-
Kelch样环氧氯丙烷相关蛋白-1(Kelch-like ECH-associated protein 1, Keap1)-核因子e2相关因子2(nuclear factor erythroid 2 related factor 2, Nrf2)系统已被证实是应对氧化应激的重要防御机制,调控该系统或许能成为众多疾病的有效治疗策略,激活Nrf2的策略已经在心血管疾病、呼吸系统疾病和神经退行性疾病等得到初步的验证[1]。
1999年,在一项双杂交筛选试验中,Keap1被鉴定为一种抑制Nrf2转录活性的蛋白质,对亲电试剂敏感[2]。Keap1的结构包括5个不同的结构域:N末端区域(n-terminal region,NTR)、BTB结构域(bric-a-brac,BTB)、间插区IVR区域(linker intervening region,IVR)、双甘氨酸重复序列结构域(double glycine repea,DGR)以及C末端区域(c-terminal region,CTR)。Keap1通过BTB结构域招募E3连接酶复合物亚基Cullin-3,并促使Nrf2进行泛素化。同时,Keap1的两个分子与底物Nrf2的单个分子结合,Keap1通过Kelch结构域和Nrf2结合,每个Kelch 结构域都与Nrf2蛋白中的2个基序之一结合,称为ETGE和DLG基序[3]。
Nrf2由基因NFE2L2编码,Nrf2存在7个高度保守的功能域Neh1至Neh7(Nrf2-ECH同源性),N末端Neh2结构域是抑制蛋白Keap1的结合位点。Neh1允许Nrf2与抗氧化反应元件(antioxidant response elements,ARE)识别并结合。Neh3位于Nrf2的羧基末端,作为反式激活结构域激活下游相关基因的转录。Neh4和Neh5代表转录激活区,通过与cAMP反应元件结合蛋白(cyclic-AMP response binding protein, CREB)相互作用来调节Nrf2转录。Neh6区域是Keap1独立的调控区域,参与Keap1替代降解途径,Neh7结构域与视黄酸X受体α相互作用,从而抑制Nrf2[4]。
Nrf2主要受Keap1调控,在正常情况下,由于E3泛素连接酶Keap1的存在,大部分Nrf2蛋白的水平在细胞质中保持较低水平。当细胞暴露于氧化应激时,Keap1的半胱氨酸残基被修饰,由Keap1介导的Nrf2泛素化被抑制。随后,Nrf2稳定,易位进入细胞核,诱导一系列靶基因的表达。这个调控系统被称为Keap1-Nrf2系统,游离和新合成的Nrf2易位到细胞核后,与一种小肌肉腱膜纤维肉瘤癌基因同源物(Maf)蛋白异二聚化,形成的异二聚体识别ARE,即Nrf2靶基因调控区域中存在的增强子序列,对于招募转录关键因子至关重要[5]。然而,Nrf2诱导剂能够与其他蛋白质上的半胱氨酸结合,共价Nrf2激活剂存在潜在的脱靶效应,因此临床前研究的重点是开发蛋白质-蛋白质相互作用(protein–protein interaction, PPI)和蛋白水解靶向嵌合体(proteolysis-targeting chimeras, PROTACs)以提高特异性[6]。
-
炎症是人体对各种刺激因素(例如某些化学物质、感染和组织损伤)的生理反应,这种防御机制涉及多种介质,引发相关反应。Keap1和Nrf2被认为是氧化应激的关键调节因子,它们通过抑制氧化应激和炎症反应保护细胞[7]。尽管Keap1-Nrf2通路在炎症中的分子作用机制尚未明确,但有些研究已经揭示了Keap1-Nrf2通路在炎症不同途径的相互作用。
炎症调控中,Nrf2与核因子-κB(nuclear factor kappa-B, NF-κB)之间存在串扰。NF-κB是一个转录因子家族,在炎症中起着至关重要的作用。由形成复合物的不同蛋白质的二聚体组成,哺乳动物中有五个NF-κB家族成员:RelA/p65、RelB、c-Rel、p50(NF-κB1)和p52(NF-κB2)[8]。在典型的NF-κB激活途径中,NF-κB被其抑制蛋白,包括IkB家族成员和其他含有ankirin重复序列的调节因子,以无活性形式隔离在细胞质中。在特定刺激如促炎细胞因子、PAMPs、氧化应激和生长因子等作用下,IKK(IκB激酶复合物)中的 IκB激酶β(IKKβ)被激活,进而磷酸化IκB蛋白,导致其泛素化和蛋白酶体降解。典型途径依赖于IKKβ和支架蛋白NEMO(NF-κB essential modulator, NEMO)主要引发IκB-α的磷酸化以及含有p65的异源二聚体的核易位。在非经典NF-κB通路中,特定细胞因子家族成员激活IKKα,导致p100磷酸化并生成p52/RelB复合物[9,10]。NF-κB作为关键调控因子之一,诱导炎症基因表达,包括促炎细胞因子、趋化因子、粘附分子、环氧合酶-2(cyclooxygenase-2, COX-2)和诱导型一氧化氮合酶(inducible nitric oxide sythase, iNOS)的转录[11]。
当前,Keap1-Nrf2通路和NF-κB在细胞中的相互作用主要涉及以下几个方面:①Keap1通过泛素化降解IKKβ,从而抑制NF-κB的活性;②Nrf2可以阻止IκB-α蛋白酶体降解,进而抑制NF-κB核易位;③NF-κB复合物的一个亚基p65与Nrf2的转录共激活因子CREB结合蛋白(CREB binding protein, CBP)竞争,因此,p65的高表达水平降低CBP对Nrf2转录的可用性,优先转录NF-κB,驱动基因Nrf2通过与CBP竞争来抵消NF-κB驱动的炎症反应;④Nrf2通过降低细胞内活性氧(reactive oxygen species, ROS)水平,抑制氧化应激介导的NF-κB活化[12];⑤Ras相关C3肉毒毒素底物1(ras-related C3 botulinum toxin substrate 1, Rac1)诱导 NF-κB,调节Nrf2,此过程是独立于Keap1,随后Nrf2抑制NF-κB,形成调节反馈环路[13]。
Keap1-Nrf2/HO-1在炎症中扮演着关键角色,HO-1是一种抗炎蛋白,其主要作用是催化血红素降解和释放抗炎因子以防止游离血红素引发的毒性。Nrf2对HO-1的表达进行调节,抑制氧化应激,减少ROS的产生,发挥抗炎作用[14]。
Nrf2是一种关键的抗炎蛋白,它通过调控炎症介质和酶的表达来发挥其抗炎作用。这包括:①细胞因子和趋化因子:Nrf2可以抑制促炎细胞因子和趋化因子的过度产生,如IL-6、IL-1β、TNF-α等。②细胞黏附分子:Nrf2可以抑制细胞间黏附分子-1(intercellular cell adhesion molecule-1,ICAM-1)和血管细胞黏附分子-1(vascular cell adhesion molecule-1, VCAM-1)等细胞黏附分子的表达,从而限制炎症细胞向炎症组织的迁移和浸润。③基质金属蛋白酶(matrix metalloproteinase, MMP):Nrf2/HO-1轴可以抑制基质金属蛋白酶-9(matrix metalloproteinase 9, MMP-9)和基质金属蛋白酶-7(matrix metalloproteinase 7, MMP-7)表达,有利于炎症性肠病的治疗。④COX-2和iNOS:Nrf2可以抑制COX-2和iNOS等促炎基因的表达,从而减轻炎症反应。总之,Nrf2在炎症过程中发挥关键作用,通过调控多种炎症介质和酶的表达,抑制炎症反应[15]。
此外,相关研究表明,Nrf2的激活可以降低ROS诱导的结构性蛋白3(NOD-like receptor thermal protein domain associated protein 3, NLRP3)炎性小体的转录,从而抑制NLRP3炎性体的激活。NF-κB、Nrf2和NLRP3炎症小体之间存在相互关系。抑制Nrf2会降低NF-κB活性,从而减轻炎症,而Nrf2的激活可以抑制NF-κB和NLRP3的表达,抑制炎症反应[16]。
-
天然产物来源的Keap1-Nrf2信号通路抑制剂在炎症性疾病治疗中具有广泛的应用前景,其中,萜类、黄酮类、生物碱类和醌类化合物在药理作用方面取得了显著的研究成果。这些发现为天然产物在炎症性疾病治疗中的潜在应用提供了有力支持,为炎症性疾病的治疗提供了多样化的选择。
-
研究表明,木香内酯通过激活Nrf2抗氧化途径降低细胞内ROS,并通过靶向NF-κB和Nrf2通路改善溃疡性结肠炎(ulcerative colitis, UC)[17]。人参皂苷Rb1直接与Keap1结合并促进其泛素化,导致Nrf2水平升高,可减少动脉粥样硬化斑块形成及氧化应激和炎症反应[18]。在神经退行性疾病方面,鼠尾草酸通过激活Keap1-Nrf2转录通路引发的2期酶诱导发挥抗氧化、抗炎和神经保护作用,进而减弱NLRP3激活,在阿尔茨海默病(alzheimer’s disease, AD)、帕金森病(parkinson's disease, PD)中具有潜在应用[19]。
-
黄酮类化合物在各种疾病状态下表现出显著的药理作用。例如,牡荆素在肾脏炎症疾病中显示出明显的疗效。它通过激活Nrf2/HO-1通路,增加谷胱甘肽过氧化酶4(glutathione peroxidase 4, GPX4)的表达,抑制脂质过氧化和铁死亡[20]。橙皮苷具有抗氧化活性和心脏保护作用,通过降低ROS和丙二醛(malondialdehyde, MDA)的水平,以及增加抗氧化标志物,如超氧化物歧化酶(superoxide dismutase, SOD)、过氧化氢酶(catalase, CAT)和谷胱甘肽的水平,减少炎症肿瘤坏死因子的释放,从而保护心脏免受毒性损害[21]。黄酮木脂素能保护小鼠免受LPS诱导的急性肺损伤(acute lung injury, ALI),依赖于抗氧化防御性Nrf2通路的上调,从而抑制LPS激活的促炎介质分泌、NLRP3炎症小体和MAPK/NF-κB信号通路[22]。
-
生物碱类化合物对多种疾病表现出重要的药理作用。例如,氧小檗碱对LPS诱导的ALI具有显著的保护作用,通过HO-1信号通路以及抑制p65的易位,增强抗氧化防御基因的表达,促进Keap1的降解和Nrf2的核转位来产生保护作用[23]。芸香碱可以通过激活Nrf2核易位和抑制HCT116细胞中活性氧的产生,增强Nrf2介导的抗氧化反应,进而抑制炎症性肠病[24]。此外,胡椒碱衍生物HJ105在AD治疗中通过抑制Keap1-Nrf2相互作用,上调核Nrf2,从而显示出神经保护作用[25]。
-
醌类化合物是一类经过深入研究的Nrf2诱导剂,其在哮喘和其他炎症性疾病中显示出重要的药理作用。具体而言,棘皮色素A(echinochrome A, EchA)作为一种天然醌,能够通过Michael受体基团的氧化转变为亲电醌,并与Keap1反应性的硫醇结合,上调Nrf2水平,发挥抗氧化和抗炎作用[26]。另一种醌类化合物大叶茜草素(mollugin, MLG)具有抗肿瘤和抗炎活性。MLG能够下调巨噬细胞在LPS或肿瘤坏死因子刺激下诱导的炎症反应,并通过激活Nrf2抗氧化途径来降低细胞内活性氧水平。此外,MLG还能够有效地与转化生长因子β活化激酶1(transforming growth factor-β activated kinase-1, TAK1)和Keap1结合,参与抑制TAK1-NF-κB/MAPKs和MLG介导的Nrf2激活[27]。
-
受到共价Nrf2激活剂显著效果的启示,研究人员已经设计和鉴定出许多直接作用于Keap1-Nrf2 PPI界面的非共价分子,期望在选择性、稳定性和安全性方面有所提升。这些分子主要可以分为几类,包括多肽类、萘磺酰胺类、四氢异喹啉类、三唑类、吲哚类。
-
Keap1通过Kelch结构域和Nrf2结合,每个Kelch结构域都与Nrf2蛋白中的ETGE和DLG基序之一结合[3]。针对这种蛋白质-蛋白质相互作用,研究者对肽抑制剂特别感兴趣,因为它们在结构上与Keap1相互作用的Nrf2表位相似。Keap1上的亲水结合位点需要带电和极性序列与Nrf2实现高亲和力相互作用,这导致细胞通透性成为一个挑战。已报道的大量线性或环状肽,尽管有部分用于增强细胞通透性,但在细胞测定中活性不足或活性显著降低,这种有限的细胞活性阻碍了Nrf2类似肽作为有效Keap1-Nrf2抑制剂的应用。Iegre等[28]提出了一种双组分肽吻合策略,以快速访问靶向Keap1-Nrf2相互作用的各种受限和功能化的肽。其中,含有脂肪酸标签的P8-H肽与Keap1表现出纳摩尔亲和力,并能有效诱导ARE基因的转录。通过Cys依赖性接合方法,可以轻松地开发具有不同功能的Nrf2肽。研究发现,肽P8-H是迄今为止已知的最有效的Keap1-Nrf2肽抑制剂。为了改善肽类化合物的活性、细胞通透性,邹季花[29]等设计了一系列以Nrf2为基础的9-mer Ac-LDEETGEFL-NH2的线性肽。在此基础上构建了一种环状结构,即通过将线性肽与Keap1结合的晶体结构相结合。这种创新设计的环状9-mer,称为ZC9,其与Keap1的结合Kd值为51 nmol/L,显示出高效的选择性及亲和力。ZC9的独特性体现在其优良的物理化学性质,如良好的水溶性(>38 mg/ml)及细胞渗透性。在体外实验中,特别是在LPS诱导的氧化损伤和急性肺损伤模型中,ZC9表现出显著的剂量反应逆转活性且无明显毒性。这些研究为肽模拟物的设计提供了有用的起点,并为了解Keap1-Nrf2通路激活机制提供了新的见解。
-
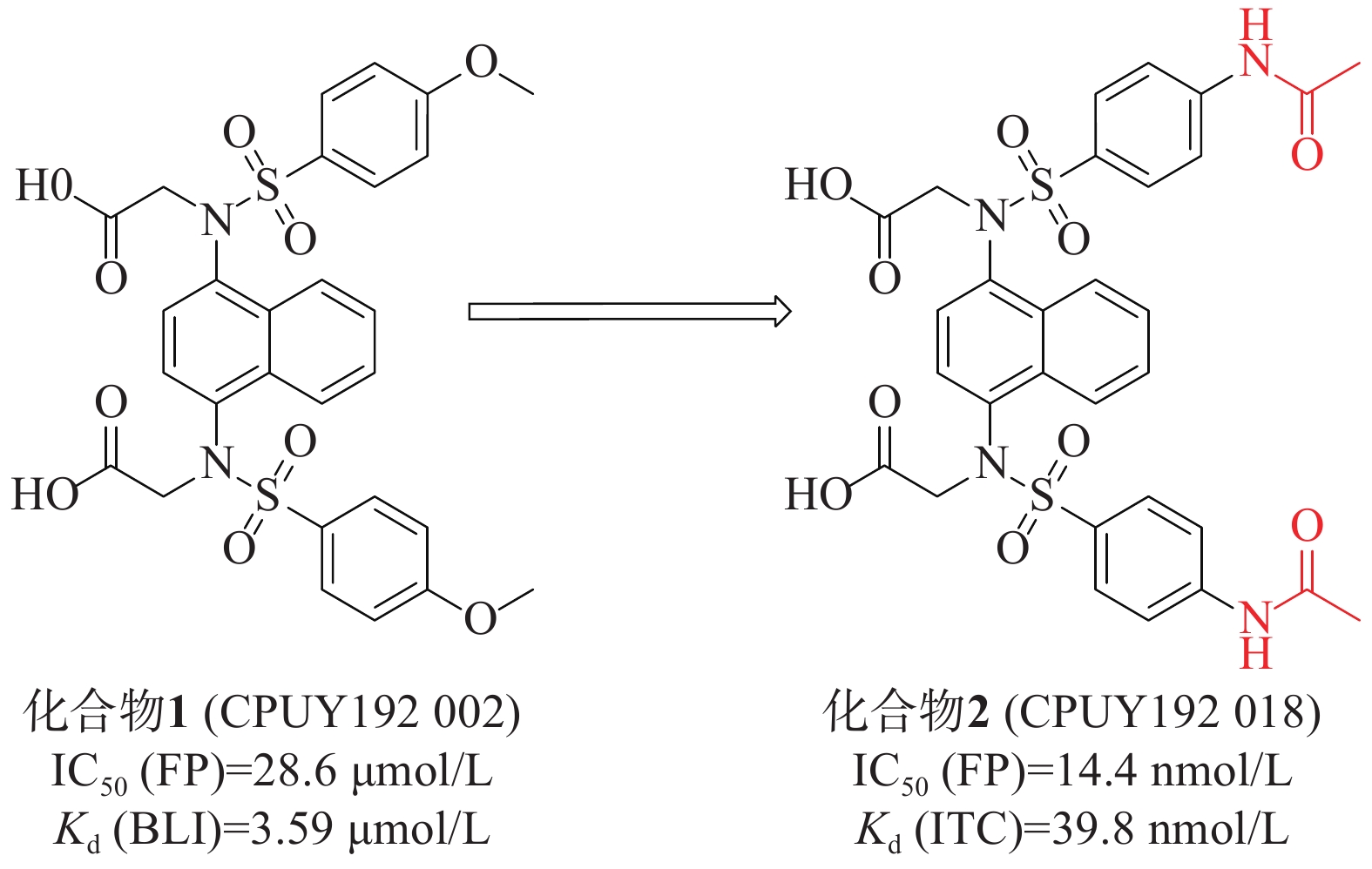
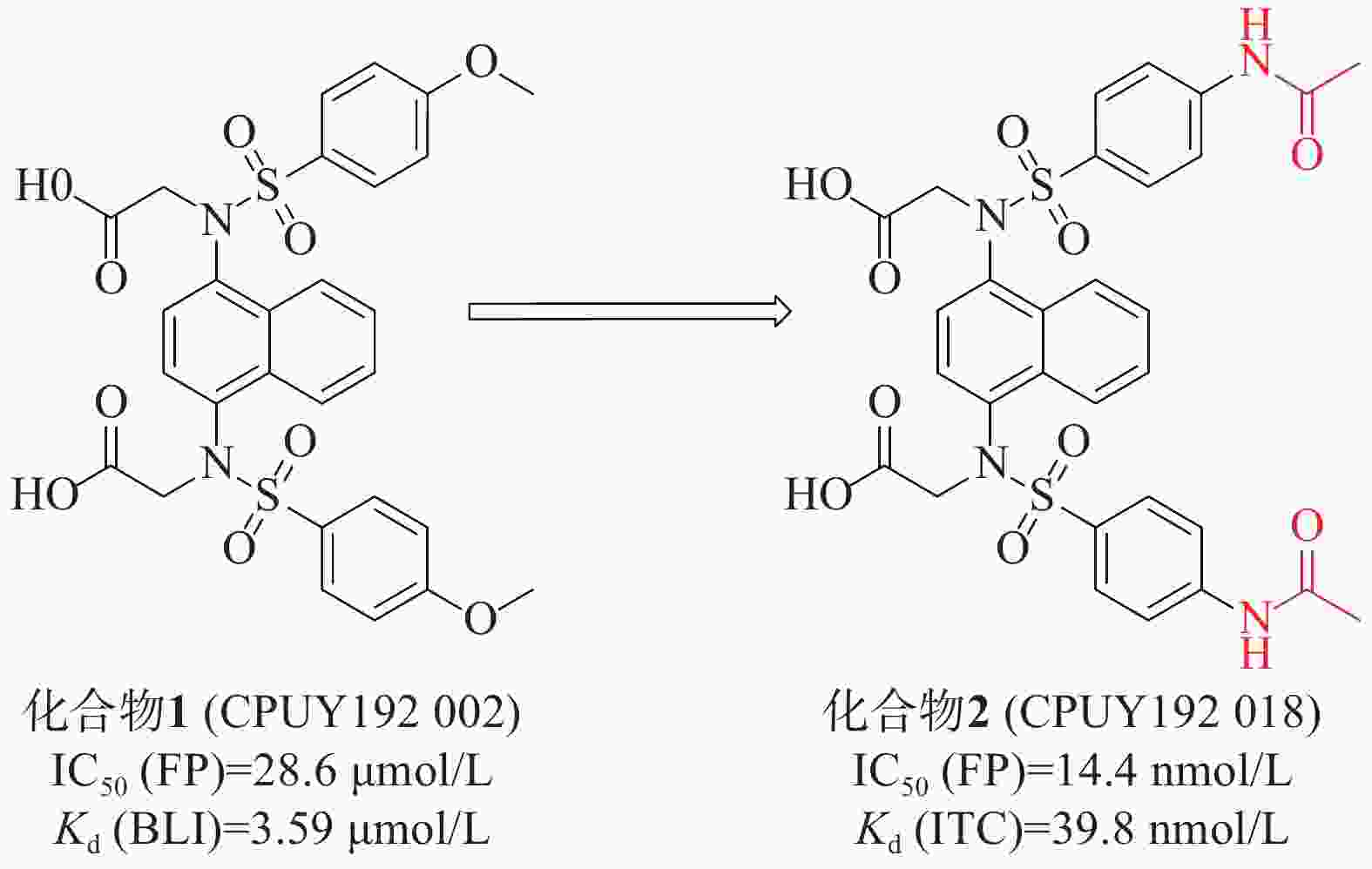
姜正羽课题组[30]发现并鉴定了第一个在纳摩尔浓度下有效的Keap1-Nrf2抑制剂化合物 1(CPUY192002),具有对称萘磺酰胺结构。通过荧光偏振法(fluorescence polarization, FP)测得该化合物的IC50值为28.6 μmol/L,生物膜层干涉(BLI)技术测得其与Keap1的结合力Kd值为3.59 μmol/L。随后,通过结构改造对CPUY192002进行溶解度优化,将结构中的甲氧基替换成乙酰氨基得到具有纳摩尔浓度的对称性分子2(CPUY192018)(IC50=14.4 nmol/L),见图1。用等温滴定量热法(isothermal titration calorimetry, ITC)测定化合物对Keap1 Kelch结构域亲和力Kd值为 39.8 nmol/L,研究表明,后者具有更好的理化性质。化合物2在体外和体内都显示出有效的Nrf2活化作用,并且已被证明在LPS诱导的慢性肾脏炎症中有抑制作用[31]。
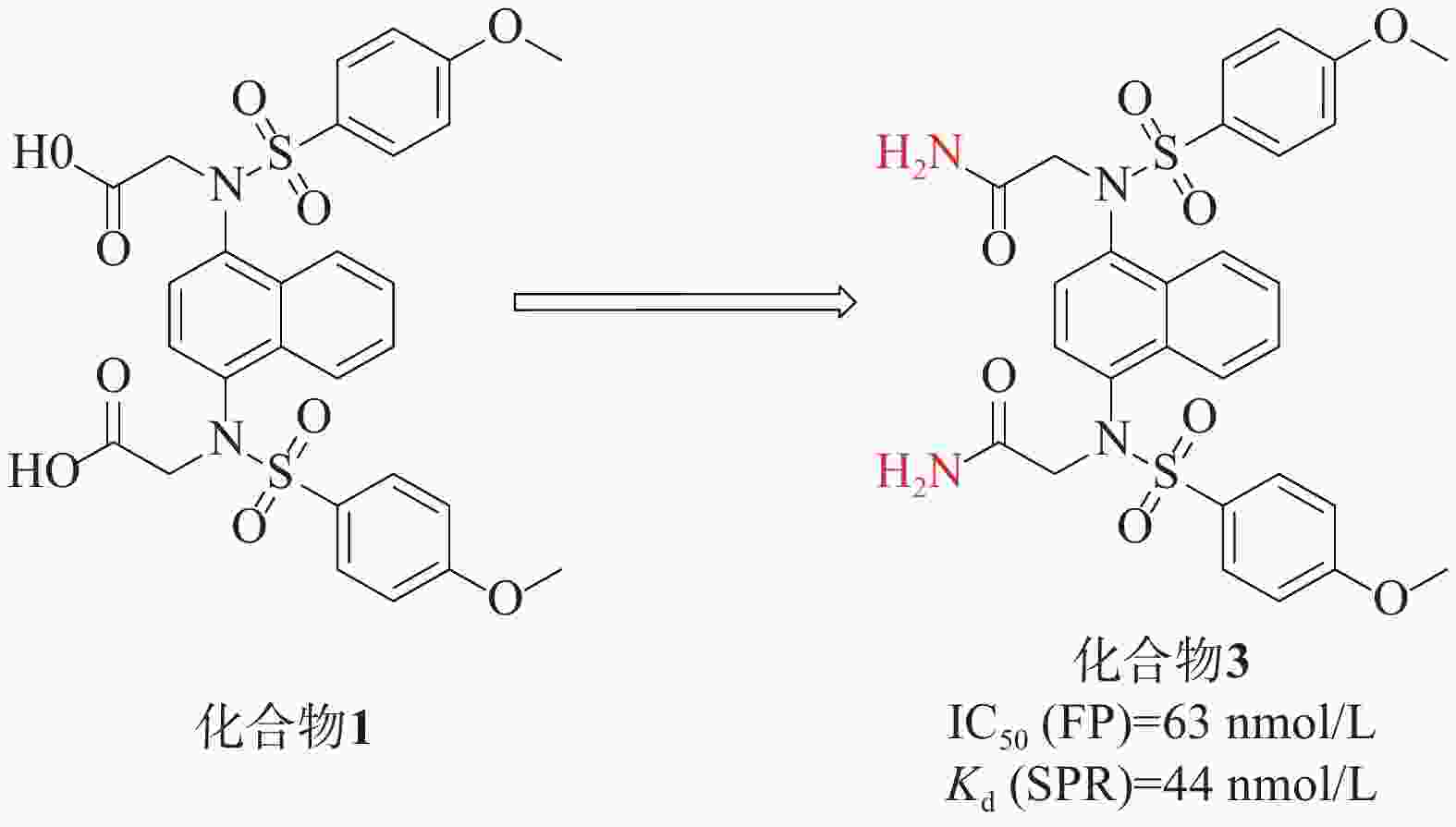
Jain[32]等人在对化合物1进行研究时,特别关注了磺胺连接区的改造。他们发现,将羧酸基团替换为二烷基对活性并没有显著效果。然而,一个关键的突破是将羧酸替换成酰胺基团得到化合物3(IC50=63 nmol/L),见图2。表面等离子共振(surface plasmon resonance, SPR)实验测得化合物3的靶点亲和力Kd值为44 nmol/L,这个改动在化合物3与Kelch络合物的X射线结构中得到了证实,化合物3中的二乙酰胺与Asn 414的酰胺侧链形成两个强氢键,同时与Ile 461的骨架羰基也形成了氢键。这一结构变化使得化合物3具有了较低的负电荷和更高的配体效率。这些改进的取代基推测可能增强了化合物3与Kelch结构域之间的亲和力,从而提高了其在该蛋白靶点上的生物活性。
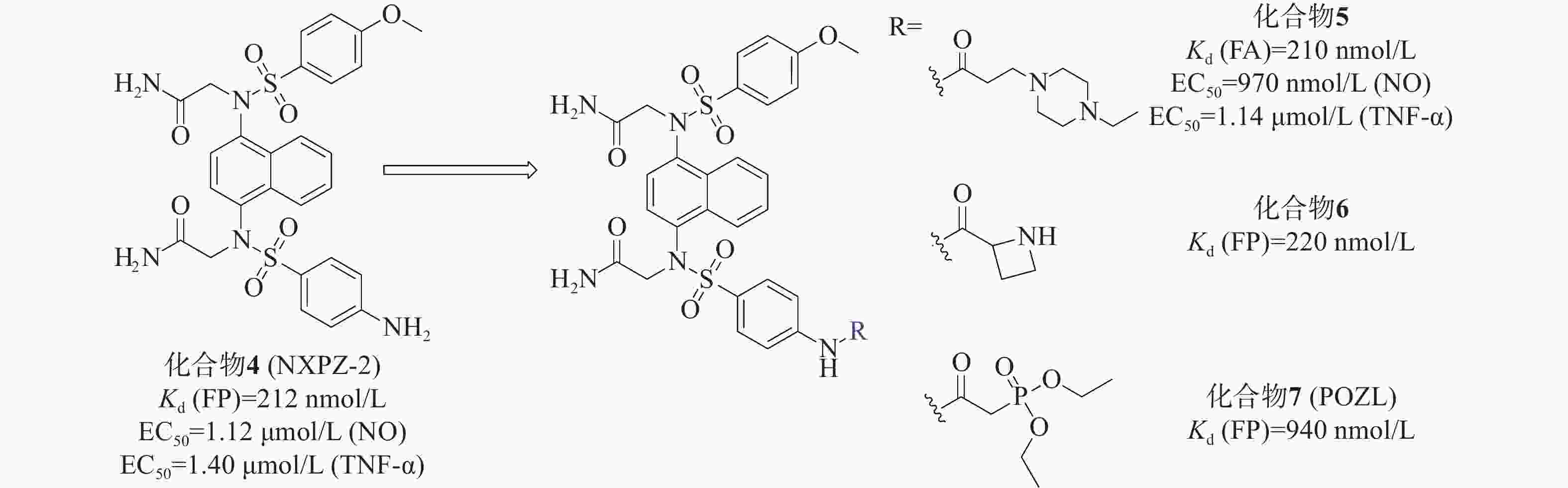
我们小组[33]先前报道的化合物4(NXPZ-2)(Kd=212 nmol/L)是在化合物3的结构基础上,将其结构中的一个甲氧基替换为氨基而得到的一种有效的Keap1-Nrf2 PPI抑制剂,见图3。NXPZ-2的高疏水性和对称性限制了其溶解度。基于首次确定的Keap1 Kelch结构域与NXPZ-2的晶体复合物,对NXPZ-2进行了进一步的结构优化,发现了不对称的萘磺酰胺[34]。降低二氨基萘的对称性是提高药物活性及溶解度的一种策略。通过在砜基的一侧引入不同的取代基,得到不对称的二氨基萘,这被证实是改善化合物体内性能的一种有效策略。
本课题组设计将链状烷烃连接到对称萘磺酰胺NXPZ-2的氨基苯基处。体外实验结果显示,结合活性随着碳链长度的增加而逐渐降低。针对溶解度较好的取代基进行了进一步的实验,结果表明,将取代基在链上多延伸两个碳,并在尾部连接哌嗪基团,得到了活性最好的化合物5[35]。荧光各向异性实验(fluorescence anisotropy, FA)测得其Kd值为210 nmol/L,其活性优于NXPZ-2,并具有较低的细胞毒性(CC50>100 μmol/L)。此外,研究团队还在NXPZ-2的氨基苯基处引入了一系列单取代氨基酸片段和伪氨基酸片段,评价了其蛋白抑制活性、水溶性和体外/体内抗炎活性。在一项新的探索中,首先评估了氨基酸在氨基苯基位置的适宜性。通过FP评价6个衍生物对Keap1-Nrf2 PPI抑制活性,并检测细胞毒性。结果显示,这6个衍生物对Keap1-Nrf2 PPI有较强的抑制作用,Kd值为250~630 nmol/L,与母药NXPZ-2一致,未见明显的细胞毒性。在进一步优化中,发现含(R)-偶氮环烷基团的化合物6(Kd =220 nmol/L)的抑制活性最高,远高于对映体和外消旋体。实验结果显示,大部分化合物能显著降低促炎细胞因子TNF-α和IL-6的水平。其中,化合物6的体外结合活性与NXPZ-2相似,具有较好的抗炎活性。进一步的抗炎实验表明,化合物6能显著减轻LPS诱导的ALI小鼠模型的炎症反应,并触发Nrf2核易位。
另外,研究团队还设计合成了一种新型的含有二氨基萘的磷酸酯化合物POZL[36]。磷酸基团的引入是调节分布系数和提高类药性质的经典药物设计策略,适用于克服NXPZ-2的局限性。磷酸二酯化合物POZL显示出最强的效力,其Kd值为940 nmol/L。研究结果表明,POZL处理能增加Nrf2的表达和核易位,抑制β-位点淀粉样前体蛋白裂解酶-1的表达,从而降低β-淀粉样蛋白的产生,改善AD小鼠的认知缺陷。
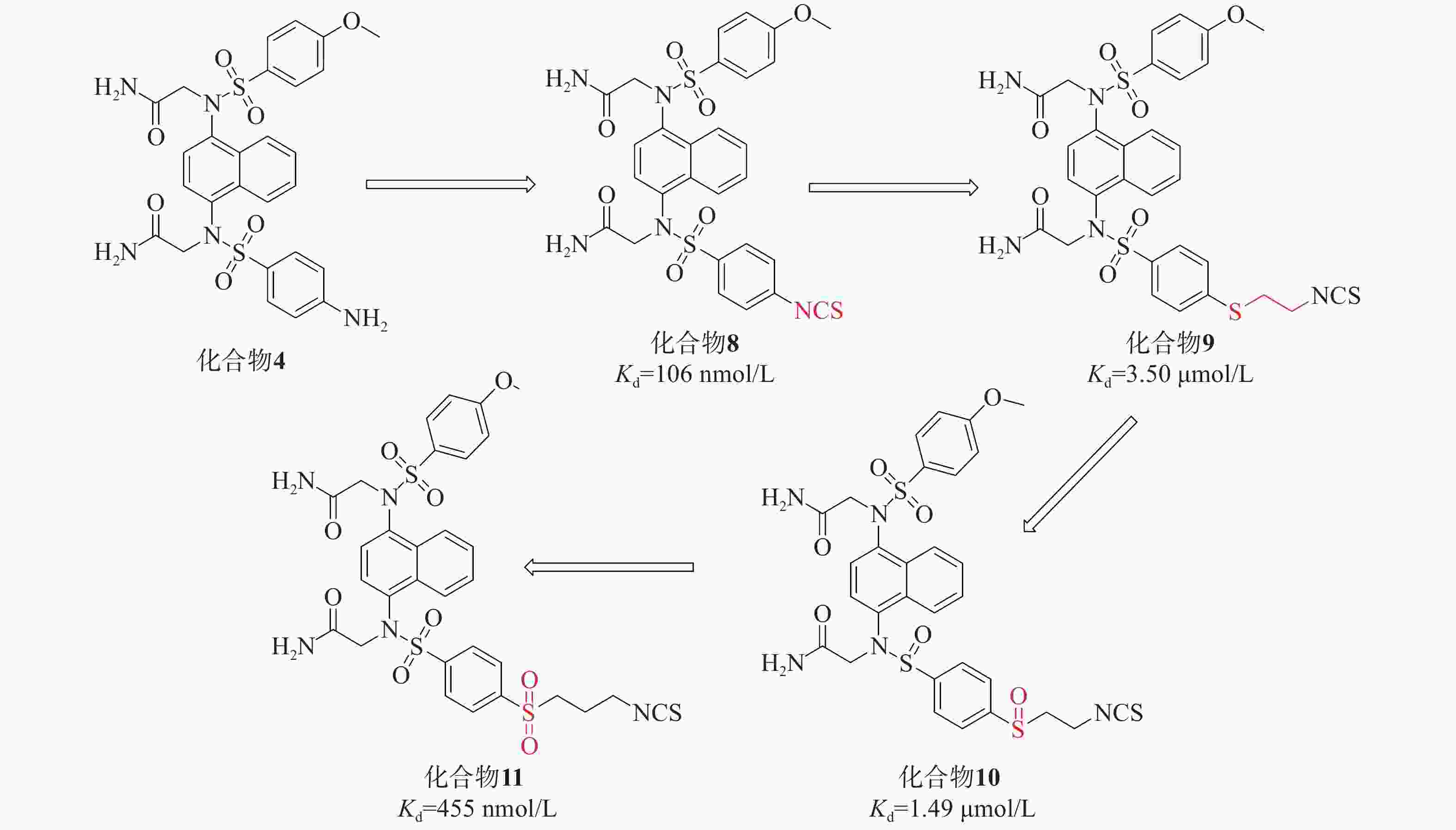
在此基础上,采用分子杂交策略,结合NXPZ-2和Nrf2活化剂萝卜硫素(sulforaphen, SFN),合成了一系列新型含异硫氰酸酯的萘磺酰胺与硫醚、亚砜和砜部分结构的化合物8~11,见图4。这些新化合物具有较好的Keap1-Nrf2 PPI抑制活性和低细胞毒性。其中,化合物11在LPS诱导的腹膜巨噬细胞模型中表现出优异的抗炎活性,通过触发Nrf2核易位减轻肺损伤。
首先,研究引入了SFN的异硫氰酸酯基团取代NXPZ-2的氨基,得到化合物8(Kd=106 nmol/L),其抑制活性高于NXPZ-2。随后,引入含有硫醚的碳链结构,得到化合物10的活性(Kd=1.49 µmol/L)比相应的化合物9(Kd=3.5 µmol/L)高2倍。进一步尝试氧化衍生物的硫原子,得到含亚砜的化合物,其中,化合物11(Kd=455 nmol/L)表现出较好的活性。对这些新化合物进行抗炎活性评价,发现它们能显著抑制ROS和NO的产生以及促炎细胞因子TNF-α的表达。在LPS诱导的ALI小鼠模型中,化合物11明显减轻了炎症和肺损伤,并触发Nrf2核易位。
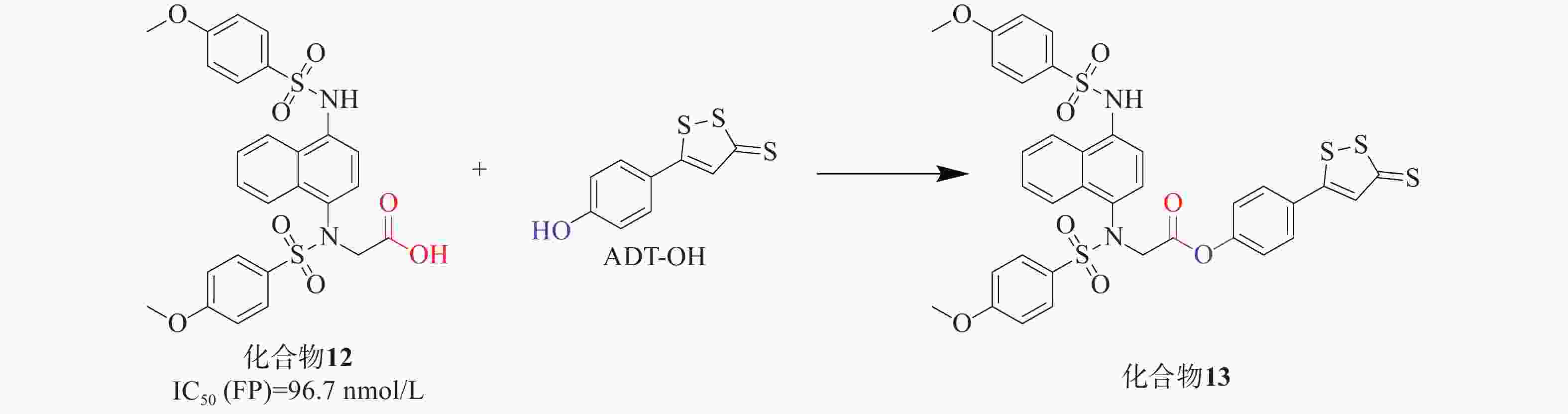
UC是一种病因不明的特发性炎症性疾病,可能与肠道炎症和氧化应激有关。Keap1-Nrf2通路为UC治疗提供了有效的防御机制,硫化氢(H2S)也显示出相似且相关的生物学功能。H2S作为重要的内源性气体信号分子,它通过促进活性氧的清除进行抗氧化,有助于修复受损组织并促进结肠炎的消退[37]。通过酯接头将Keap1-Nrf2 PPI抑制剂与一个成熟的H2S供体部分连接,合成了一系列杂交衍生物。化合物12[38]与是一种有效的Keap1-Nrf2 PPI抑制剂(IC50=96.7 nmol/L),其被选中进一步修饰。通过酯键将化合物12与H2S供体ADT-OH衍生物偶联,设计制备了一系列新的杂化分子,见图5。其中,化合物13[39]被确定为疗效最好的候选药物。实验结果表明,化合物13能有效缓解硫酸葡糖钠诱导的结肠炎,提高对氧化应激的防御能力,比母体药物更有效。总体而言,Keap1-Nrf2抑制剂和H2S供体的协同组合具有治疗溃疡性结肠炎的潜力,分子杂交可能是治疗多因素炎症性疾病的一种有前途的策略。
-
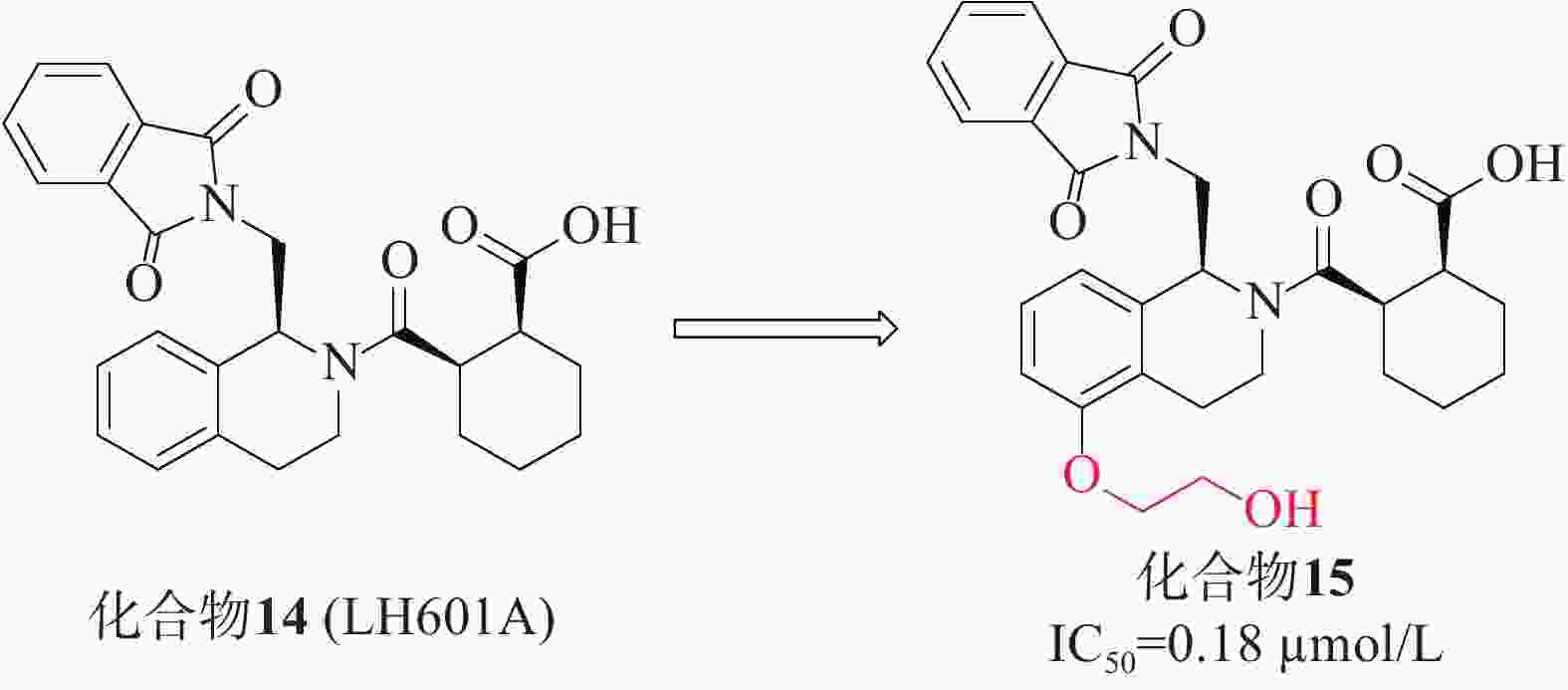
第一个非共价Keap1-Nrf2抑制剂是在2013年通过高通量筛选发现的四氢异喹啉类化合物14(LH601A)[40],见图6,是唯一具有活性的非对映体,FP实验中测得其亲和力IC50值为2.3 µmol/L,SPR实验测得其与Keap1的结合活性Kd值为1.0 µmol/L。在细胞水平上,LH601A可以诱导Nrf2核易位,上调Nrf2靶基因和蛋白。化合物14和早期报道的萘磺酰胺类抑制剂的一个共同特征是存在至少1个羧酸基团。迄今为止,用酰胺取代萘磺酰胺的2个酸性基团已合成得到多种高活性的Nrf2抑制剂,因此,研究人员尝试用四氮唑、甲酯、酰胺等替代酸或制备两性离子类似物,结果表明其抑制活性减弱或消失,证明该酸性官能团不可取代[41]。后续的优化研究在LH601A的第5位引入了乙二醇得到了化合物15(IC50 = 0.18 µmol/L),其中,化合物15的结合亲和力增加了4倍,且对MDCK-MDR1细胞具有通透性[42]。在非酸性四氢异喹啉(THIQ)的第5位引入乙二醇或羟基取代基允许在P3口袋处参与额外的氢键相互作用,从而产生迄今为止已知的最有效的THIQ Keap1结合剂。然而,由于含酸化合物常见的血脑屏障穿透性差,因此在中枢神经系统适应症方面,该类抑制剂的开发仍需持续的研究。
-
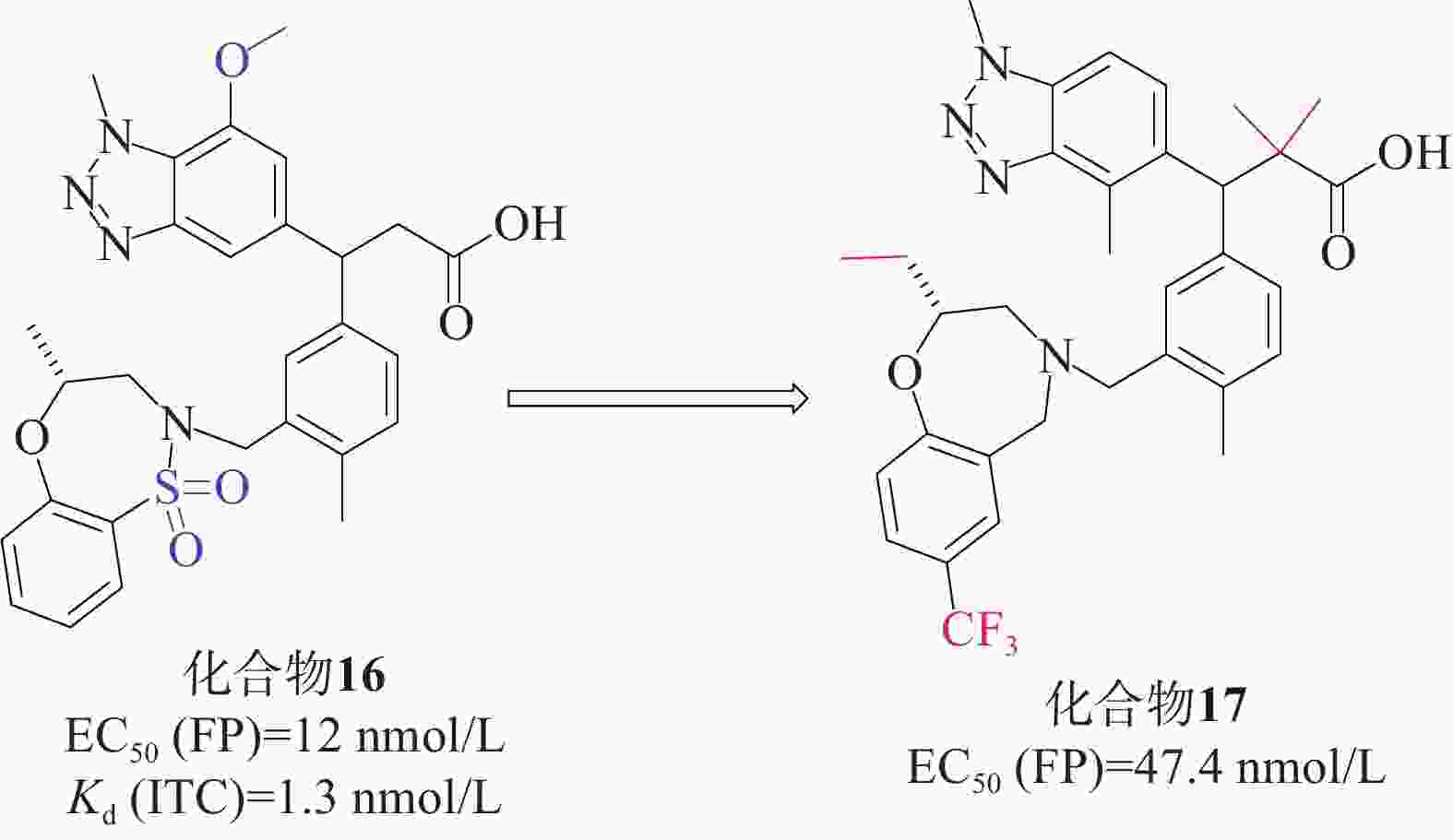
Tong等[43]首次成功运用基于片段的方法直接针对Keap1-Nrf2相互作用进行研究。他们通过X射线晶体学筛选,确定了Keap1-Nrf2结合口袋中的3个“热点”。这一方法帮助他们获得了一种具有纳摩尔亲和力的Keap1分子结合的先导化合物。研究人员发现,将苯并三唑部分直接连接到苯丙酸的α碳上,能与Keap1的侧链Tyr525形成堆叠相互作用,同时满足Gln530和Ser555与双齿受体基序的氢键要求。经过一系列的结构优化,研究人员得到了候选化合物16[44](EC50=12 nmol/L,Kd=1.3 nmol/L),见图7。化合物16满足从片段筛选中鉴定的三点药效团(酸、芳香受体和磺胺),并对Keap1 Kelch结构域具有很高的亲和力,因此,它可以作为基于细胞和体内研究的理想工具化合物。在人肺上皮细胞系中也证实了化合物16增加NQO1活性的能力,并且对支气管上皮细胞无细胞毒性。在此基础上,根据结构活性关系对候选化合物16进行了优化。成功筛选出一种具有显著抑制Keap1-Nrf2相互作用的化合物17[45](EC50=47.4 nmol/L)。
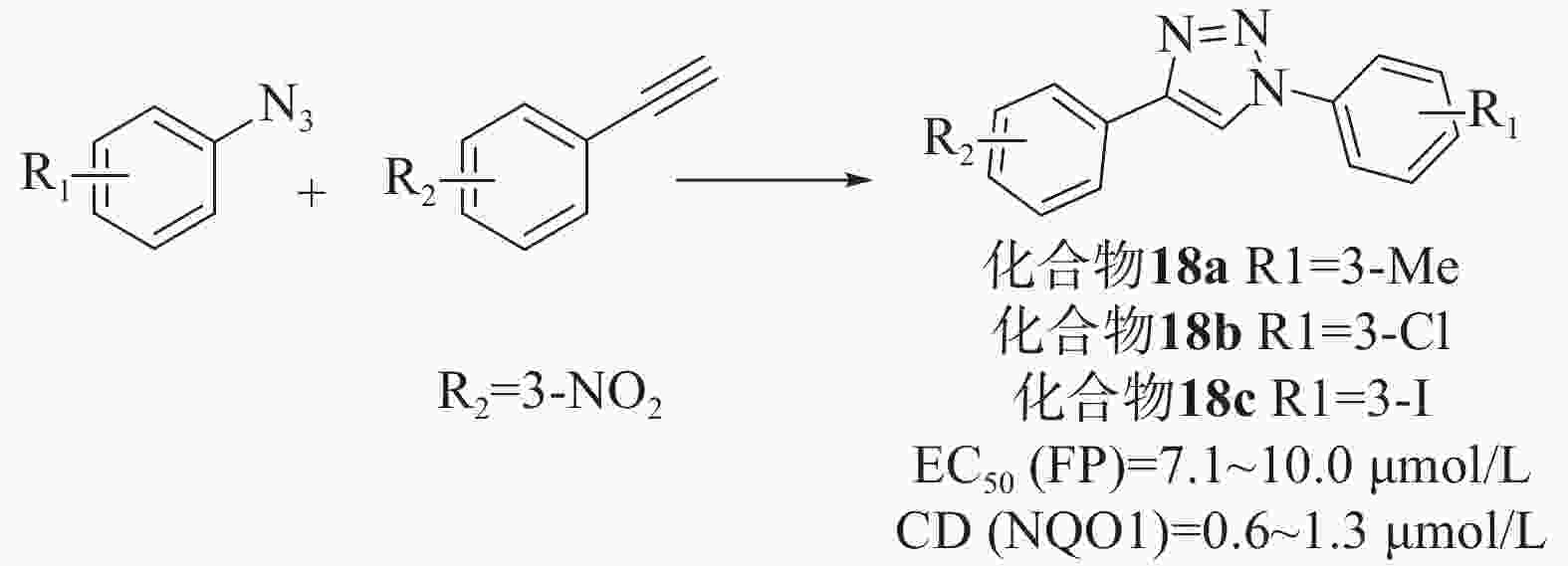
Wells等[46]通过对接和虚拟筛选设计了一系列基于1,4-二芳基-1,2,3-三唑支架的配体。首先,通过乙基和叠氮苯中间体反应合成了36个三唑类文库,在固定剂量的FP测定和已建立的比色法NQO1诱导法(在Hepa1c1c7小鼠肝癌细胞中评估Nrf2依赖性转录)中,除苯基4位带有对甲酰胺的化合物外,所有化合物均能够在10 μmol/L浓度下破坏Keap1-Nrf2相互作用,是具有显著效力的苯甲酸衍生物。然而,只有6种化合物在细胞测定中显示出Nrf2诱导能力,其中4个带有4-(3-硝基苯基)三唑基序,所以选择4-苯基三唑部分的间硝基取代作为第二代文库。第二代文库由1-乙基- 3-硝基苯和一系列3位取代基合成的,大部分化合物在 FP 和 NQO1 诱导测定中表现出良好的效活性。为了检验四唑部分作为羧酸同分异构体的影响,制备了第三个文库,并以1-乙基-3-三苯和之前筛选的小系列3-取代苯肼为原料,制备了一系列1,2,3-三唑化合物。虽然FP实验中有两种化合物表现出显著的活性,但NQO1诱导能力测试普遍较差,表明它们不是有效的Nrf2诱导剂。剂量-反应评价实验结果表明,除了叔丁基和硫甲基取代基外,其他取代基活性均良好。其中,化合物18a、18b和18c显示出优越的活性[EC50=7.1~10.0 μmol/L、CD(NQO1)=0.6~1.3 μmol/L],见图8。同时,透析测定证明了碘取代化合物的可逆结合,也证实了该化合物通过破坏Keap1-DLG而不是Keap1-ETGE相互作用来发挥作用。
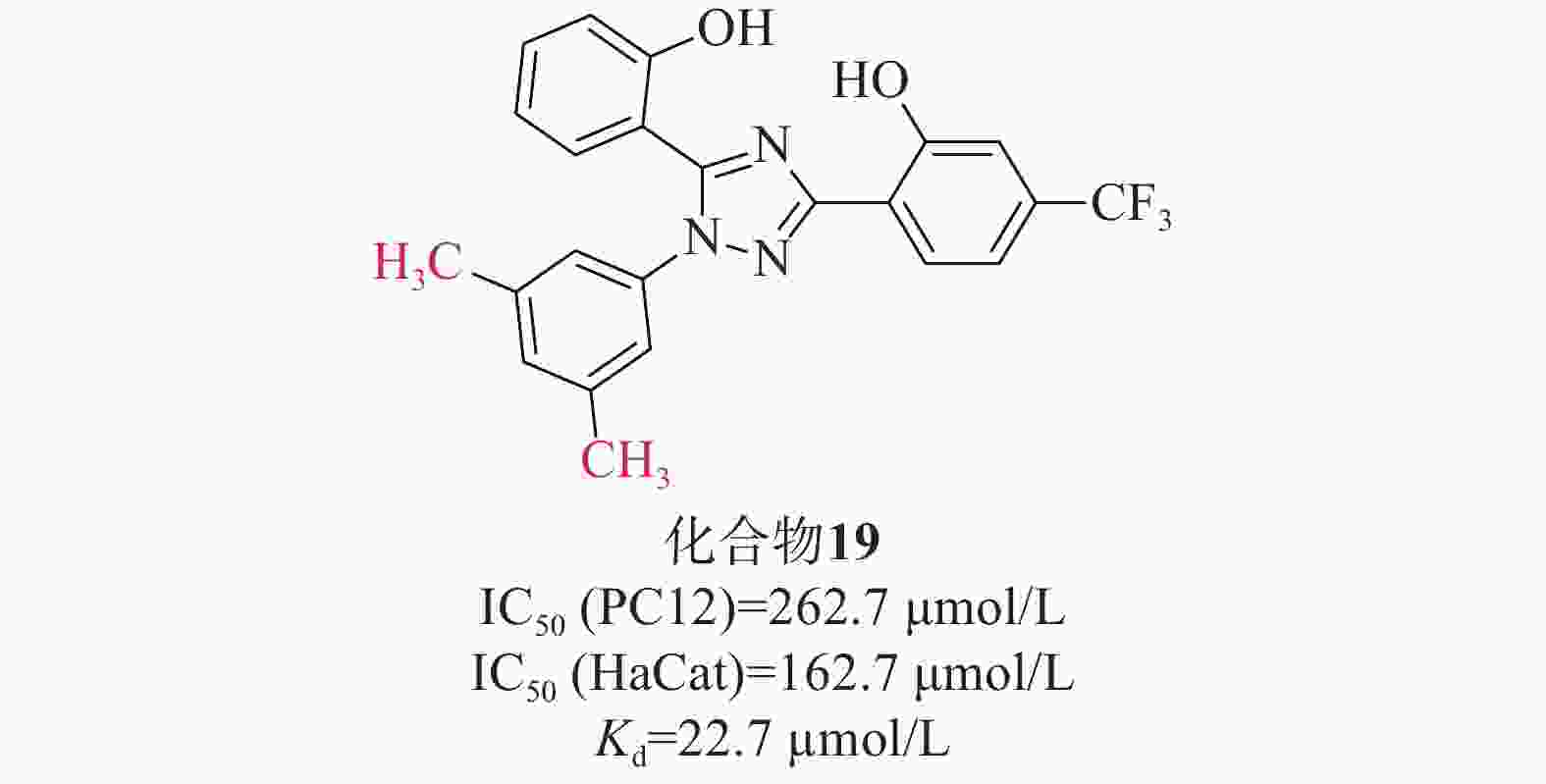
张静夏课题组[47]考虑到酚类取代基噁二唑可能对脑缺血损伤具有潜在的保护作用,而三唑的电子性质与噁二唑相似,故将1,2,4-噁二唑核心替换为1,2,4-三唑,以获得酚取代基三唑。为了增加血脑屏障透过率,在骨架中添加苯基以提高其亲脂性,合成了一系列在苯基上具有不同取代基的化合物进行进一步的探索。硝普钠(sodium nitroprusside,SNP)作为刺激物诱导PC12细胞凋亡,可以模拟缺血性脑卒中神经元氧化应激。首先通过SNP诱导的PC12细胞凋亡模型初步筛选了这些化合物的神经保护活性。在4位被单基团取代时,含烷基的化合物可能发挥更好的神经保护作用,其次是含卤素的化合物;3位取代基优于2位或4位取代基;此外,同时占据位置3和5位可能有利于神经保护作用。进一步研究对有前途的化合物在PC12和HaCaT细胞上的细胞毒性评估其安全性[IC50(PC12)=262.7 μmol/L、 IC50(HaCat)=162.7 μmol/L]。两项测试表明(图9),化合物19(Kd=22.7 µmol/L)突出的神经保护作用以及对PC12和HaCaT细胞的低毒性,被选为进一步实验化合物。化合物19通过提高SOD水平和降低MDA的产生来缓解体内氧化应激。此外,Western Blot检测发现化合物19促进Nrf2的核易位及上调Nrf2依赖性酶的表达水平,SPR实验表明,化合物19能与Keap1 Kelch结构域结合。
-
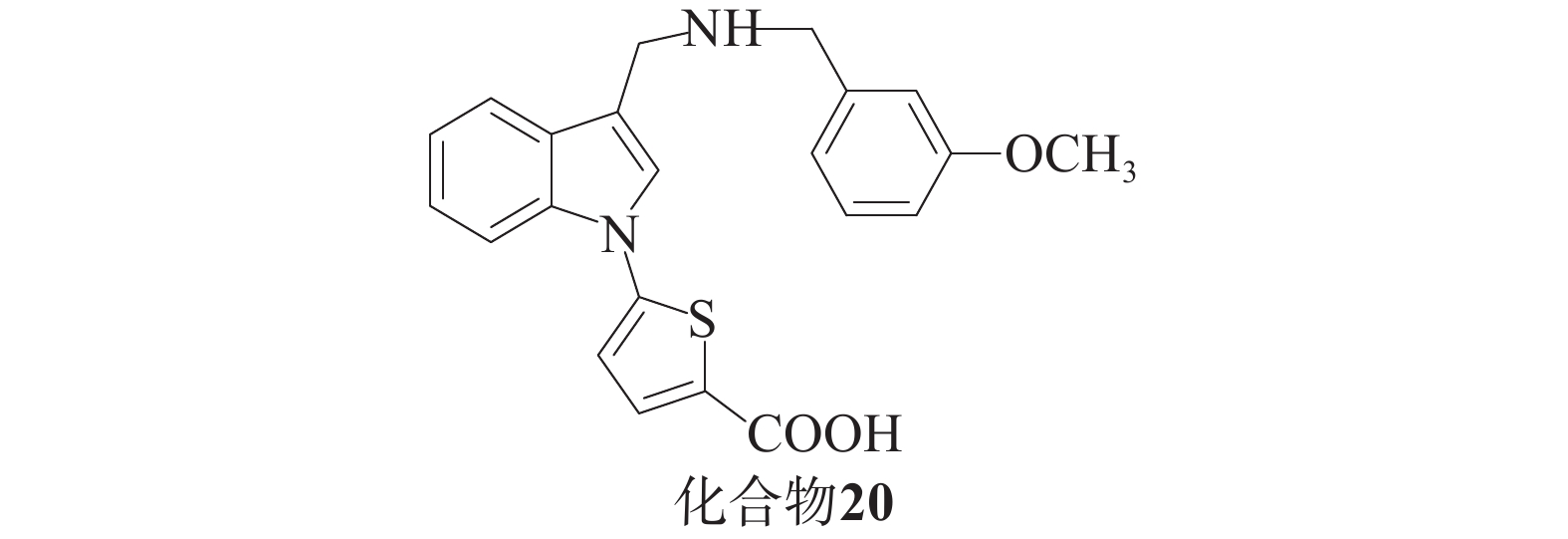
Barbara课题组[48]基于分子建模和亚结构搜索方法,发现了一系列化合物。酸性抑制剂与带有羧基的Keap1-Nrf2相互作用以阴离子形式存在于中性水溶液中,这会阻碍细胞易位过程。为了解决这个问题,研究人员尝试使用四唑环或硝基替代羧基,获得了对Keap1具有高亲和力的化合物,并在基于细胞的实验中展现出更高的活性(图10),其中,化合物20被发现是活性最高的吲哚衍生物。这一系列化合物的生物学实验结果表明,在对Nrf2及其下游靶ARE基因NQO1和TKT编码的两种酶的表达实验中,化合物20活性更强。在细胞毒性实验中,GI50值大于5 µmol/L,毒性最小。
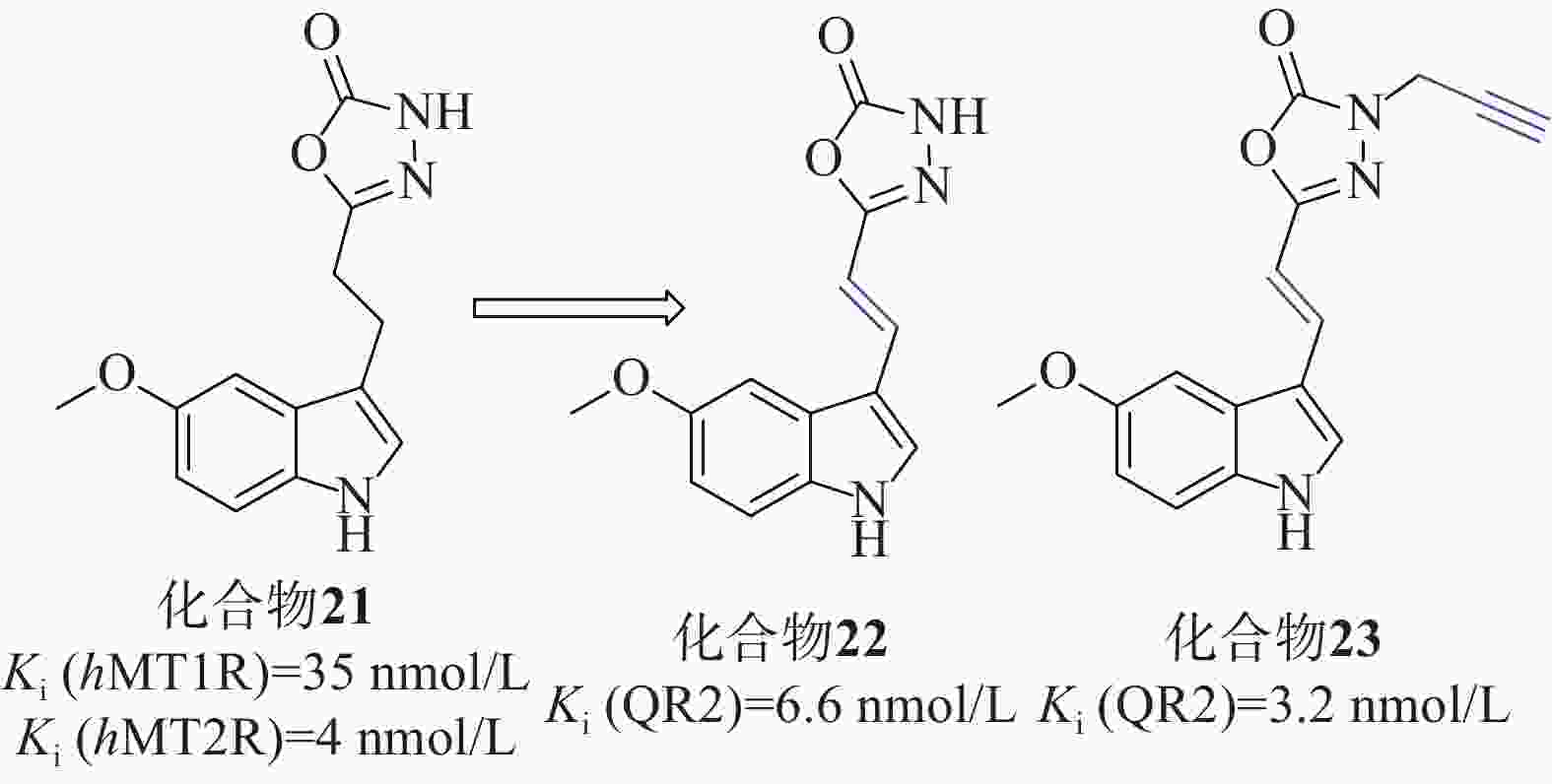
Clara等[49]以褪黑激素类似物化合物21[Ki (hMT1R)=35 nmol/L、Ki (hMT2R)=4 nmol/L]为起始化合物,在噁二唑酮氮上加入不同取代基、用噁二唑胺取代噁二唑酮环、在连接物中插入双键、用二氢萘环或萘环代替吲哚,对起始分子的每个部分进行了修改,得到了一系列类似物,见图11。
在氧自由基吸收能力(oxygen radical absorbance capacity, ORAC)的评估测试中,所有吲哚衍生物的ORAC值优良,证明与杂环上3号取代基的性质无关,用二氢萘或萘芯代替吲哚对ORAC测定中的抗氧化活性有害。在AREc32细胞系中,使用Nrf2依赖性荧光素酶报告基因试验来评估激活Nrf2的能力,化合物22、23的诱导效果最好,CD值分别为15.1和1.8 µmol/L,Ki(QR2)值分别为6.6、3.2 nmol/L,实验表明连接体中存在吲哚核和双键对于实现Nrf2的良好诱导至关重要。实验人员为了评估分子与Keap1的Kealch结构域相互作用的能力,进行了SPR实验,以著名的Keap1结合剂ML334作为内参,在产生的10个共振单元的SPR信号变化中,3个化合物SPR响应值等于或大于ML334,4个化合物SPR值较小,可初步判定化合物22、23对Nrf2的激活可能是由于这些分子与Keap1结合所致。
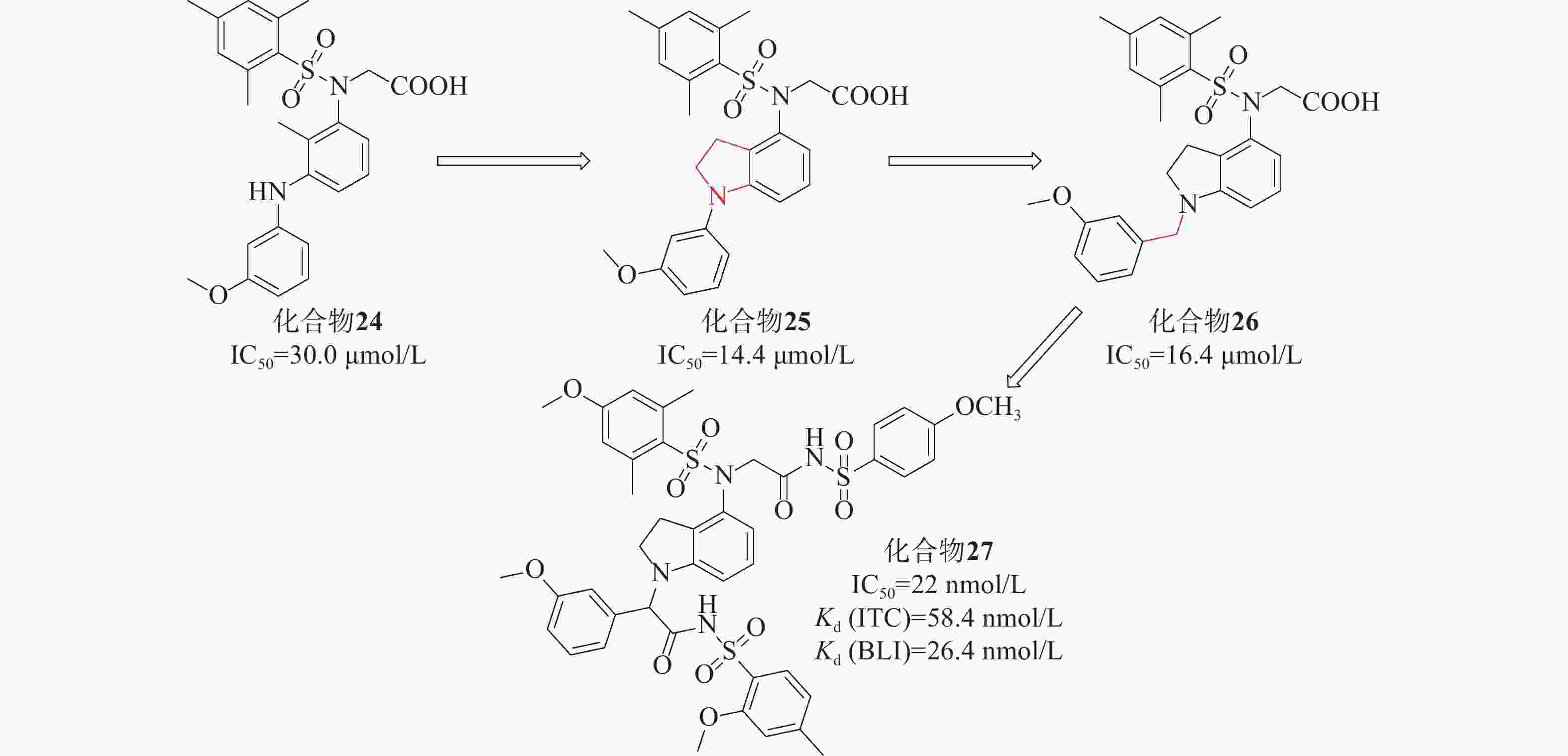
姜正羽课题组[50]基于已有结构,用苯环取代了萘环。然后,在核心苯环上引入一个小的疏水基团3-甲氧基苯胺,采用FP法研究了化合物核心苯环上不同取代基对其活性的影响,发现在其核心苯环引入2位甲基的化合物24(IC50=30.0 μmol/L)的效力最高。课题组假设化合物24的核心苯环可能占据P3口袋以稳定结合构象。将仲胺与核心苯环的甲基部分环化得到化合物25(IC50=14.4 μmol/L),与化合物24相比,其活性提高了大约2倍。随后基于化合物25的结构,对其吲哚环结构、苯磺酰胺片段上取代基以及吲哚环和苯基环之间的连接部分进行探索。系统地评估了化合物26苄基部分的不同取代基对活性的影响,发现原来的3-甲氧基是最优选择。化合物26与Keap1的分子对接结合模式发现羧酸部分插入P2子口袋,2,4,6-三甲基取代苯基环占据疏水性口袋P5,苄基部分占据极性P1子口袋。最终以亚甲基类似物化合物26(IC50=16.4 μmol/L)进行下一步优化,见图12。
该课题组进一步对化合物26进行设计优化,最后得到了活性最高的化合物27 [IC50=22 nmol/L、Kd(ITC)=58.4 nmol/L 、Kd(BLI)=26.4 nmol/L],在体外显著激活Nrf2调控的细胞保护防御系统。体内实验证明,化合物27对LPS诱导的H9C2细胞损伤具有细胞保护作用。该课题组将吲哚环为核心代替萘支架设计的一系列化合物,促进了Keap1-Nrf2 PPI抑制剂的研发。
-
在众多Keap1-Nrf2通路抗炎药物中,有3种药物近年来应用最广泛:富马酸二甲酯、齐墩果烷三萜、甲基巴多索隆(bardoxolone methyl, CDDO-Me)。研究人员不断推动其临床进展,与此同时,其他药物也相继进入临床试验,如表1所示。
表 1 药物在临床试验中的应用
临床试验编号 试验标题 试验药物 适应证 NCT06319339 Nrf2激活对周围动脉疾病患者大血管功能、微血管功能、腿部功能和步行能力的影响 富马酸地罗西美 外周动脉闭塞性疾病 CTIS2023- 506795 -27-00富马酸二甲酯对肾上腺脊髓神经病患者的影响:一项多中心、安慰剂对照、Ⅱb/Ⅲ期试验 富马酸二甲酯 神经系统疾病/肾上腺脑白质营养不良 NCT05959759 富马酸二甲酯治疗颅内未破裂动脉瘤:双盲随机对照试验 富马酸二甲酯 颅内动脉瘤炎症 CTR20232004 单中心、开放、单次给药试验评价硝酮嗪片在肾功能不全和健康受试者中药代动力学特征 硝酮嗪 肾功能不全糖尿病肾病 NCT05811949 富马酸二甲酯对多发性硬化症认知表现、灰质和丘脑病理学的影响:相关性研究。 富马酸二甲酯 多发性硬化症 NCT05798520 一项2部分、多中心、随机、盲法、主动控制的Ⅱ期研究,依次评估 BIIB091 单一疗法和BIIB091联合富马酸地罗昔美治疗复发型多发性硬化症参与者的安全性和有效性 富马酸地罗西美,BIIB-091 复发性多发性硬化 NCT05718375 CU01- 1001 治疗2型糖尿病肾病蛋白尿患者 24 周疗效评估和安全性评估的多中心、随机、双盲、安慰剂对照、平行Ⅱb 期临床试验CU-01 白蛋白尿2型糖尿病肾
脏病CTR20221451 一项旨在评价Diroximel Fumarate(BIIB098)用于亚太地区成年复发型多发性硬化受试者中的安全性和耐受性以及药代动力学的开放性、单臂、多中心、Ⅲ期研究 富马酸地罗西美 复发性多发性硬化 NCT05083923 一项开放标签、单臂、多中心、Ⅲ期研究,旨在评估富马酸二羟甲基(BIIB098)在亚太地区复发性多发性硬化症患者中的安全性、耐受性和药代动力学 富马酸地罗西美 复发性多发性硬化 NCT04948606 一项前瞻性观察性研究,评估真实世界环境中富马酸二羟甲基嘧啶的治疗持久性、安全性、耐受性和有效性(EXPERIENCE-CA+IL研究) 富马酸地罗西美 复发性多发性硬化 NCT04890353 免疫调节剂富马酸二甲酯对急性缺血性中风的影响 富马酸二甲酯 急性缺血性卒中 NCT04890366 免疫调节剂富马酸二甲酯与阿替普酶联合治疗急性缺血性中风 富马酸二甲酯 急性缺血性卒中 CTR20210206 多中心、随机、双盲、安慰剂平行对照试验探索性研究硝酮嗪治疗 2 型糖尿病肾病患者的有效性和安全性 硝酮嗪 2型糖尿病肾病 NCT04702997 评估甲基巴多索隆在有快速进展风险的慢性肾病患者中的安全性、耐受性和疗效的Ⅱ期试验 甲基巴多索隆 慢性肾病 NCT04657926 使用夹竹桃苷和丹皮酚(APPA)治疗膝关节骨性关节炎的安慰剂对照、双盲、随机试验 APPA 膝关节炎 CTR20202126 硝酮嗪治疗肌萎缩侧索硬化症有效性和安全性的多中心、随机、双盲、安慰剂对照临床研究 硝酮嗪 肌萎缩侧索硬化 NCT04468165 通用延迟释放富马酸二甲酯(Sclera® 或Marovarex®, Hikma)在治疗中东和北非地区复发缓解型多发性硬化症的常规医疗实践中的有效性和安全性 富马酸二甲酯 复发-缓解型多发性硬化 CTR20192413 多中心、随机、双盲、平行、安慰剂对照试验研究硝酮嗪片治疗2型糖尿病肾病患者的有效性和安全性 硝酮嗪 2型糖尿病肾脏病 NCT04292080 富马酸二甲酯的长期分析,以减缓地理萎缩区域的增长 富马酸二甲酯 地图样萎缩年龄相关性黄斑变性 NCT04263610 一项开放标签、随机、Ⅳ 期研究,评估 Tildrakizumab 在对富马酸二甲酯治疗无反应的中度至重度慢性斑块状银屑病患者中的疗效和安全性(TRANSITION) 富马酸二甲酯替瑞奇珠单抗 斑块状银屑病 NCT04221191 法国患者支持计划(PSP)OroSEP 中包含的缓解-复发性多发性硬化症(RR-MS)患者的富马酸二甲酯(Tecfidera®)持久性研究 富马酸二甲酯 复发-缓解型多发性硬化 NCT04142749 一项评估奥替普拉疗效和安全性的多中心、随机、双盲、安慰剂对照、平行、Ⅲ期临床试验 奥替普拉 肝硬化代谢功能障碍相关的脂肪肝病 NCT04125745 CXA-10在肺动脉高压中的安全性和有效性试验的Ⅱ期开放标签研究 10-硝基油酸 肺动脉高压 NCT04072861 在健康志愿者和慢性肾病 3/4 期受试者中使用 RBT-9进行的Ⅰb期剂量递增研究 Stannous protoporphyrin (Renibus Therapeutics) 慢性肾病 -
许多神经系统疾病的发生与氧化应激密切相关,神经炎症是由创伤、缺氧、中毒、感染等因素引发的中枢及周围神经系统的炎症,可能导致神经元变性及退化,成为阿尔茨海默病、帕金森病、中风等神经系统疾病及神经损伤的关键病理过程。因此,抑制炎症对于预防和治疗神经系统疾病具有重要意义。
备受关注的富马酸二甲酯于2014年就被渤健公司批准用于治疗复发型多发性硬化症[51],富马酸地罗西美也针对复发性多发性硬化进行了临床试验(NCT05798520)。越来越多的研究者关注到SFN对神经系统的保护作用,研究结果证实了SFN在急慢性神经退行性病变和神经发育疾病等神经系统疾病的预防和辅助治疗方面具有巨大应用潜力。SFN通过经典途径调控Nrf2通路,即通过化学修饰Keap1半胱氨酸残基(主要为Cys151)阻止Keap1与Nrf2结合,从而抑制Nrf2的泛素化和降解,实现Nrf2的积累以及依赖Nrf2调控的下游基因转录增强[52],减轻炎症反应。
-
研究表明,Nrf2作为一种重要的抗氧化基因,对心血管系统具有保护作用。已在动物实验中得到证实,Nrf2敲除小鼠较正常小鼠心血管事件发生率增加,提示Nrf2表达与各种心血管疾病的发生发展有着重要联系[21]。Nrf2在心血管疾病中的作用机制复杂且多样。在动脉粥样硬化中,Nrf2通过抑制氧化低密度脂蛋白的摄取和泡沫细胞的形成,降低斑块形成的风险[53]。在缺血再灌注损伤与心肌梗死中,Nrf2能够减轻氧化应激和炎症反应,从而减少心肌损伤和梗死面积[54]。此外,研究还发现,在心血管疾病中Nrf2的表达受到多种因素的调控,如Keap1、p27蛋白、钙离子稳态调节等。这些调控因子在不同的心血管疾病中发挥的作用也各有差异,进一步揭示了Nrf2在心血管疾病中具有广泛的应用前景。
-
在肾缺血再灌注、慢性肾脏疾病和糖尿病肾病等不同类型的肾脏疾病中,Nrf2的激活都发挥着关键性的作用。它主要通过以下两个方面的作用来发挥其生理功能:第一,Nrf2的活化能够上调肾组织中抗氧化蛋白的表达,如硫氧还蛋白、HO-1和GCL等,从而清除体内的过量活性氧。第二,Nrf2还能够调控NF-κB,抑制肾组织中炎症介质的表达,如细胞因子、趋化因子、黏附分子、COX-2和iNOS等[55]。炎症介质的过度表达会导致肾脏组织的损伤和炎症反应,Nrf2通过抑制这些炎症介质的表达,能够发挥肾脏保护作用。
由Reata制药公司开发的CDDO-Me是另一种广为人知的Nrf2激活剂,可强烈激活Keap1-Nrf2系统[56]。研究表明,CDDO-Me的开发有望为各种类型肾脏疾病患者提供有前景的新治疗方法。如今评估CDDO-Me在慢性肾病患者中的安全性、耐受性和疗效的临床试验已经进展到Ⅱ期(NCT04702997)。
-
先前的研究发现,Nrf2激活能够防止酒精诱导的氧化应激和肝脏中游离脂肪酸的积累,通过增加参与抗氧化防御的基因表达和减少参与脂肪生成的基因表达来发挥肝脏保护作用[57]。刘等[58]发现,黄芩苷显著减弱代谢功能障碍相关性脂肪肝小鼠肝组织中的脂质积累、肝硬化和肝细胞凋亡,降低促炎生物标志物并增强抗氧化酶,这些酶似乎受到上调的p62-Keap1-Nrf2信号级联的调节;黄芩苷和全反式维甲酸(Nrf2抑制剂)的联合治疗显示出黄芩苷及其诱导的抗氧化和抗炎反应明显减弱了肝脏保护作用。
-
本文主要探讨了Keap1-Nrf2通路在炎症疾病中的研究进展。首先,介绍了Keap1-Nrf2通路的基本概念,包括其组成成分及激活机制。其次,阐述了Keap1-Nrf2通路调控炎症的机制,包括与NF-κB通路、HO-1之间的串扰、与炎症介质和酶的表达、炎症小体之间的关系。此外,本文还介绍了通过激活Keap1-Nrf2通路来治疗炎症的天然产物来源抑制剂、小分子抑制剂以及临床进展。
近年来,大量研究已经表明Keap1-Nrf2通路在炎症疾病中具有重要的调控作用。通过了解该通路的激活机制和调控机制,可以开发出针对该通路的治疗策略。天然产物来源的抑制剂和小分子抑制剂已经显示出潜在的治疗效果,并且在临床上取得了一些进展。然而,许多问题也不断接踵而来,亲电性的Keap-Nrf2抑制剂的非特异性、小分子Keap1-Nrf2 PPI的潜在脱靶作用、天然产物来源的抑制剂对机体的特异性和副作用都将限制它们在临床中的应用。仍然需要进一步的研究来深入了解Keap1-Nrf2通路的调控机制,并开发更具针对性和高效性的治疗方法。例如,Nrf2基因具有双重作用,既能为正常细胞提供保护,抵抗氧化应激,被认为具有肿瘤抑制功能;然而在肿瘤细胞中过度激活,通过抗氧化作用,促进肿瘤细胞的存活和增殖,表现出癌基因的特征[1]。目前迫切需要和精准的Keap1-Nrf2抑制剂来实现病理条件下Nrf2的特异性激活。
此外,可以探索新的治疗策略,如基因编辑和基因治疗、蛋白降解技术如PROTACs以及自噬靶向嵌合体(autophagy-targeting chimeras, AUTUCs)以提高Keap1-Nrf2通路的活性和特异性。同时,进一步研究Keap1-Nrf2通路与其他关键信号通路之间的相互作用,以及其在不同炎症疾病中的具体调控机制。总之,Keap1-Nrf2通路在炎症治疗中具有巨大的潜力,为炎症疾病的治疗提供了新的思路。
作者贡献:周文艳负责文献检索、数据核对及综述撰写;胡珊珊参与负责文献检索、数据核对及综述撰写;张万年、庄春林负责为综述撰写思路并对稿件进行修改和审校。
利益冲突:所有作者均不存在利益冲突。
Research progresses on Keap1-Nrf2 pathway in inflammatory diseases
-
摘要: Kelch样环氧氯丙烷相关蛋白-1-核因子e2相关因子2(Keap1-Nrf2) 通路已被证实是应对氧化应激的重要防御机制,调控该系统或许能成为众多疾病的有效治疗策略。主要探讨了Keap1-Nrf2通路在炎症疾病中的研究进展,介绍了Keap1-Nrf2通路的基本组成成分及激活机制,阐述了Keap1-Nrf2通路调控炎症与NF-κB通路、HO-1之间的影响、与炎症介质和酶的表达、炎症小体之间的关系。介绍了靶向Keap1-Nrf2通路的天然产物来源抑制剂、小分子抑制剂以及临床进展,探讨了Keap1-Nrf2通路在炎症治疗中的潜在应用价值。Abstract: The Keap1-Nrf2 pathway has been shown to be an important defense mechanism against oxidative stress, which may be an effective therapeutic strategy for many diseases. The research progresses on Keap1-Nrf2 pathway in inflammatory diseases were mainly reviewed. The basic components and activation mechanism of Keap1-Nrf2 pathway were introduced. The relationship between Keap1-Nrf2 pathway and the crosstalk between NF-κB pathway and HO-1 pathway, the expression of inflammatory mediators and enzymes, and inflammatory bodies were expounded. Natural product-derived inhibitors, small molecule inhibitors targeting Keap1-Nrf2 pathway and their clinical progress were introduced, and the potential application value of Keap1-Nrf2 pathway in the treatment of inflammation was discussed.
-
Key words:
- Keap1 /
- Nrf2 /
- mechanism of action /
- inflammation /
- inhibitor
-
表 1 药物在临床试验中的应用
临床试验编号 试验标题 试验药物 适应证 NCT06319339 Nrf2激活对周围动脉疾病患者大血管功能、微血管功能、腿部功能和步行能力的影响 富马酸地罗西美 外周动脉闭塞性疾病 CTIS2023- 506795 -27-00富马酸二甲酯对肾上腺脊髓神经病患者的影响:一项多中心、安慰剂对照、Ⅱb/Ⅲ期试验 富马酸二甲酯 神经系统疾病/肾上腺脑白质营养不良 NCT05959759 富马酸二甲酯治疗颅内未破裂动脉瘤:双盲随机对照试验 富马酸二甲酯 颅内动脉瘤炎症 CTR20232004 单中心、开放、单次给药试验评价硝酮嗪片在肾功能不全和健康受试者中药代动力学特征 硝酮嗪 肾功能不全糖尿病肾病 NCT05811949 富马酸二甲酯对多发性硬化症认知表现、灰质和丘脑病理学的影响:相关性研究。 富马酸二甲酯 多发性硬化症 NCT05798520 一项2部分、多中心、随机、盲法、主动控制的Ⅱ期研究,依次评估 BIIB091 单一疗法和BIIB091联合富马酸地罗昔美治疗复发型多发性硬化症参与者的安全性和有效性 富马酸地罗西美,BIIB-091 复发性多发性硬化 NCT05718375 CU01- 1001 治疗2型糖尿病肾病蛋白尿患者 24 周疗效评估和安全性评估的多中心、随机、双盲、安慰剂对照、平行Ⅱb 期临床试验CU-01 白蛋白尿2型糖尿病肾
脏病CTR20221451 一项旨在评价Diroximel Fumarate(BIIB098)用于亚太地区成年复发型多发性硬化受试者中的安全性和耐受性以及药代动力学的开放性、单臂、多中心、Ⅲ期研究 富马酸地罗西美 复发性多发性硬化 NCT05083923 一项开放标签、单臂、多中心、Ⅲ期研究,旨在评估富马酸二羟甲基(BIIB098)在亚太地区复发性多发性硬化症患者中的安全性、耐受性和药代动力学 富马酸地罗西美 复发性多发性硬化 NCT04948606 一项前瞻性观察性研究,评估真实世界环境中富马酸二羟甲基嘧啶的治疗持久性、安全性、耐受性和有效性(EXPERIENCE-CA+IL研究) 富马酸地罗西美 复发性多发性硬化 NCT04890353 免疫调节剂富马酸二甲酯对急性缺血性中风的影响 富马酸二甲酯 急性缺血性卒中 NCT04890366 免疫调节剂富马酸二甲酯与阿替普酶联合治疗急性缺血性中风 富马酸二甲酯 急性缺血性卒中 CTR20210206 多中心、随机、双盲、安慰剂平行对照试验探索性研究硝酮嗪治疗 2 型糖尿病肾病患者的有效性和安全性 硝酮嗪 2型糖尿病肾病 NCT04702997 评估甲基巴多索隆在有快速进展风险的慢性肾病患者中的安全性、耐受性和疗效的Ⅱ期试验 甲基巴多索隆 慢性肾病 NCT04657926 使用夹竹桃苷和丹皮酚(APPA)治疗膝关节骨性关节炎的安慰剂对照、双盲、随机试验 APPA 膝关节炎 CTR20202126 硝酮嗪治疗肌萎缩侧索硬化症有效性和安全性的多中心、随机、双盲、安慰剂对照临床研究 硝酮嗪 肌萎缩侧索硬化 NCT04468165 通用延迟释放富马酸二甲酯(Sclera® 或Marovarex®, Hikma)在治疗中东和北非地区复发缓解型多发性硬化症的常规医疗实践中的有效性和安全性 富马酸二甲酯 复发-缓解型多发性硬化 CTR20192413 多中心、随机、双盲、平行、安慰剂对照试验研究硝酮嗪片治疗2型糖尿病肾病患者的有效性和安全性 硝酮嗪 2型糖尿病肾脏病 NCT04292080 富马酸二甲酯的长期分析,以减缓地理萎缩区域的增长 富马酸二甲酯 地图样萎缩年龄相关性黄斑变性 NCT04263610 一项开放标签、随机、Ⅳ 期研究,评估 Tildrakizumab 在对富马酸二甲酯治疗无反应的中度至重度慢性斑块状银屑病患者中的疗效和安全性(TRANSITION) 富马酸二甲酯替瑞奇珠单抗 斑块状银屑病 NCT04221191 法国患者支持计划(PSP)OroSEP 中包含的缓解-复发性多发性硬化症(RR-MS)患者的富马酸二甲酯(Tecfidera®)持久性研究 富马酸二甲酯 复发-缓解型多发性硬化 NCT04142749 一项评估奥替普拉疗效和安全性的多中心、随机、双盲、安慰剂对照、平行、Ⅲ期临床试验 奥替普拉 肝硬化代谢功能障碍相关的脂肪肝病 NCT04125745 CXA-10在肺动脉高压中的安全性和有效性试验的Ⅱ期开放标签研究 10-硝基油酸 肺动脉高压 NCT04072861 在健康志愿者和慢性肾病 3/4 期受试者中使用 RBT-9进行的Ⅰb期剂量递增研究 Stannous protoporphyrin (Renibus Therapeutics) 慢性肾病 -
[1] LIN L, WU Q, LU F F, et al. Nrf2 signaling pathway: current status and potential therapeutic targetable role in human cancers[J]. Front Oncol, 2023, 13:1184079. doi: 10.3389/fonc.2023.1184079 [2] ITOH K, WAKABAYASHI N, KATOH Y, et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain[J]. Genes Dev, 1999, 13(1):76-86. doi: 10.1101/gad.13.1.76 [3] DINKOVA-KOSTOVA A T, KOSTOV R V, CANNING P. Keap1, the cysteine-based mammalian intracellular sensor for electrophiles and oxidants[J]. Arch Biochem Biophys, 2017, 617:84-93. doi: 10.1016/j.abb.2016.08.005 [4] BELLEZZA I, GIAMBANCO I, MINELLI A, et al. Nrf2-Keap1 signaling in oxidative and reductive stress[J]. Biochim Biophys Acta Mol Cell Res, 2018, 1865(5):721-733. doi: 10.1016/j.bbamcr.2018.02.010 [5] YAMAMOTO M, KENSLER T W, MOTOHASHI H. The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis[J]. Physiol Rev, 2018, 98(3):1169-1203. doi: 10.1152/physrev.00023.2017 [6] ADINOLFI S, PATINEN T, JAWAHAR DEEN A, et al. The KEAP1-NRF2 pathway: Targets for therapy and role in cancer[J]. Redox Biol, 2023, 63:102726. doi: 10.1016/j.redox.2023.102726 [7] ALMUKAINZI M, EL-MASRY T A, SELIM H, et al. New insight on the cytoprotective/antioxidant pathway Keap1/Nrf2/HO-1 modulation by Ulva intestinalis extract and its selenium nanoparticles in rats with carrageenan-induced paw edema[J]. Mar Drugs, 2023, 21(9):459. doi: 10.3390/md21090459 [8] HAYDEN M S, GHOSH S. NF-κB, the first quarter-century: remarkable progress and outstanding questions[J]. Genes Dev, 2012, 26(3):203-234. doi: 10.1101/gad.183434.111 [9] SAHA S, BUTTARI B, PANIERI E, et al. An overview of Nrf2 signaling pathway and its role in inflammation[J]. Molecules, 2020, 25(22):5474. doi: 10.3390/molecules25225474 [10] OECKINGHAUS A, HAYDEN M S, GHOSH S. Crosstalk in NF-κB signaling pathways[J]. Nat Immunol, 2011, 12(8):695-708. doi: 10.1038/ni.2065 [11] LI Q T, VERMA I M. NF-kappaB regulation in the immune system[J]. Nat Rev Immunol, 2002, 2(10):725-734. doi: 10.1038/nri910 [12] GANESH YERRA V, NEGI G, SHARMA S S, et al. Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-κB pathways in diabetic neuropathy[J]. Redox Biol, 2013, 1(1):394-397. doi: 10.1016/j.redox.2013.07.005 [13] CUADRADO A, MARTÍN-MOLDES Z, YE J P, et al. Transcription factors NRF2 and NF-κB are coordinated effectors of the Rho family, GTP-binding protein RAC1 during inflammation[J]. J Biol Chem, 2014, 289(22):15244-15258. doi: 10.1074/jbc.M113.540633 [14] HUANG C Y, DENG J S, HUANG W C, et al. Attenuation of lipopolysaccharide-induced acute lung injury by hispolon in mice, through regulating the TLR4/PI3K/Akt/mTOR and Keap1/Nrf2/HO-1 pathways, and suppressing oxidative stress-mediated ER stress-induced apoptosis and autophagy[J]. Nutrients, 2020, 12(6):1742. doi: 10.3390/nu12061742 [15] AHMED S M, LUO L, NAMANI A, et al. Nrf2 signaling pathway: Pivotal roles in inflammation[J]. Biochim Biophys Acta Mol Basis Dis, 2017, 1863(2):585-597. doi: 10.1016/j.bbadis.2016.11.005 [16] HOU Y H, WANG Y T, HE Q, et al. Nrf2 inhibits NLRP3 inflammasome activation through regulating Trx1/TXNIP complex in cerebral ischemia reperfusion injury[J]. Behav Brain Res, 2018, 336:32-39. doi: 10.1016/j.bbr.2017.06.027 [17] HE J C, LIU L, LIU X J, et al. Epoxymicheliolide prevents dextran sulfate sodium-induced colitis in mice by inhibiting TAK1-NF-κB pathway and activating Keap1-NRF2 signaling in macrophages[J]. Int Immunopharmacol, 2022, 113: 109404. [18] WANG Z C, NIU K M, WU Y J, et al. A dual Keap1 and p47phox inhibitor Ginsenoside Rb1 ameliorates high glucose/ox-LDL-induced endothelial cell injury and atherosclerosis[J]. Cell Death Dis, 2022, 13(9):824. doi: 10.1038/s41419-022-05274-x [19] SATOH T, TRUDLER D, OH C K, et al. Potential therapeutic use of the rosemary diterpene carnosic acid for Alzheimer’s disease, Parkinson’s disease, and long-COVID through NRF2 activation to counteract the NLRP3 inflammasome[J]. Antioxidants, 2022, 11(1):124. doi: 10.3390/antiox11010124 [20] SONG J Y, WANG H R, SHENG J Y, et al. Vitexin attenuates chronic kidney disease by inhibiting renal tubular epithelial cell ferroptosis via NRF2 activation[J]. Mol Med, 2023, 29(1):147. doi: 10.1186/s10020-023-00735-1 [21] JIA Y X, GUO H, CHENG X Z, et al. Hesperidin protects against cisplatin-induced cardiotoxicity in mice by regulating the p62-Keap1-Nrf2 pathway[J]. Food Funct, 2022, 13(7):4205-4215. doi: 10.1039/D2FO00298A [22] HONG H W, LOU S Y, ZHENG F L, et al. Hydnocarpin D attenuates lipopolysaccharide-induced acute lung injury via MAPK/NF-κB and Keap1/Nrf2/HO-1 pathway[J]. Phytomedicine, 2022, 101:154143. doi: 10.1016/j.phymed.2022.154143 [23] AI G X, WU X Y, DOU Y X, et al. Oxyberberine, a novel HO-1 agonist, effectively ameliorates oxidative stress and inflammatory response in LPS/D-GalN induced acute liver injury mice via coactivating erythrocyte metabolism and Nrf2 signaling pathway[J]. Food Chem Toxicol, 2022, 166:113215. doi: 10.1016/j.fct.2022.113215 [24] ZHANG Y B, YAN T T, SUN D X, et al. Rutaecarpine inhibits KEAP1-NRF2 interaction to activate NRF2 and ameliorate dextran sulfate sodium-induced colitis[J]. Free Radic Biol Med, 2020, 148:33-41. doi: 10.1016/j.freeradbiomed.2019.12.012 [25] YANG X P, ZHI J K, LENG H F, et al. The piperine derivative HJ105 inhibits Aβ1-42-induced neuroinflammation and oxidative damage via the Keap1-Nrf2-TXNIP axis[J]. Phytomedicine, 2021, 87:153571. doi: 10.1016/j.phymed.2021.153571 [26] LEE S M, HU L Q. Nrf2 activation through the inhibition of Keap1-Nrf2 protein-protein interaction[J]. Med Chem Res, 2020, 29(5):846-867. doi: 10.1007/s00044-020-02539-y [27] LIU X J, SHEN X F, WANG H, et al. Mollugin prevents CLP-induced sepsis in mice by inhibiting TAK1-NF-κB/MAPKs pathways and activating Keap1-Nrf2 pathway in macrophages[J]. Int Immunopharmacol, 2023, 125: 111079. [28] IEGRE J, KRAJCOVICOVA S, GUNNARSSON A, et al. A cell-active cyclic peptide targeting the Nrf2/Keap1 protein-protein interaction[J]. Chem Sci, 2023, 14(39):10800-10805. doi: 10.1039/D3SC04083F [29] ZOU J H, YAN J Y, LU Y F, et al. Cyclic peptide Keap1-Nrf2 protein-protein interaction inhibitors: design, synthesis, and in vivo treatment of acute lung injury[J]. J Med Chem, 2024, 67(6):4889-4903. doi: 10.1021/acs.jmedchem.4c00065 [30] JIANG Z Y, LU M C, XU L L, et al. Discovery of potent Keap1-Nrf2 protein-protein interaction inhibitor based on molecular binding determinants analysis[J]. J Med Chem, 2014, 57(6):2736-2745. doi: 10.1021/jm5000529 [31] LU M C, ZHAO J, LIU Y T, et al. CPUY192018, a potent inhibitor of the Keap1-Nrf2 protein-protein interaction, alleviates renal inflammation in mice by restricting oxidative stress and NF-κB activation[J]. Redox Biol, 2019, 26:101266. doi: 10.1016/j.redox.2019.101266 [32] JAIN A D, POTTETI H, RICHARDSON B G, et al. Probing the structural requirements of non-electrophilic naphthalene-based Nrf2 activators[J]. Eur J Med Chem, 2015, 103:252-268. doi: 10.1016/j.ejmech.2015.08.049 [33] ZHANG L, XU L J, CHEN H H, et al. Structure-based molecular hybridization design of Keap1-Nrf2 inhibitors as novel protective agents of acute lung injury[J]. Eur J Med Chem, 2021, 222:113599. doi: 10.1016/j.ejmech.2021.113599 [34] WINKEL A F, ENGEL C K, MARGERIE D, et al. Characterization of RA839, a noncovalent small molecule binder to Keap1 and selective activator of Nrf2 signaling[J]. J Biol Chem, 2015, 290(47):28446-28455. doi: 10.1074/jbc.M115.678136 [35] LIU G D, HOU R L, XU L J, et al. Crystallography-guided optimizations of the Keap1-Nrf2 inhibitors on the solvent exposed region: from symmetric to asymmetric naphthalenesulfonamides[J]. J Med Chem, 2022, 65(12):8289-8302. doi: 10.1021/acs.jmedchem.2c00170 [36] SUN Y, XU L J, ZHENG D P, et al. A potent phosphodiester Keap1-Nrf2 protein-protein interaction inhibitor as the efficient treatment of Alzheimer’s disease[J]. Redox Biol, 2023, 64:102793. doi: 10.1016/j.redox.2023.102793 [37] XIE Z Z, LIU Y, BIAN J S. Hydrogen sulfide and cellular redox homeostasis[J]. Oxid Med Cell Longev, 2016, 2016:6043038. doi: 10.1155/2016/6043038 [38] LU M C, ZHANG X, ZHAO J, et al. A hydrogen peroxide responsive prodrug of Keap1-Nrf2 inhibitor for improving oral absorption and selective activation in inflammatory conditions[J]. Redox Biol, 2020, 34:101565. doi: 10.1016/j.redox.2020.101565 [39] ZHANG X, CUI K N, WANG X L, et al. Novel hydrogen sulfide hybrid derivatives of Keap1-Nrf2 protein-protein interaction inhibitor alleviate inflammation and oxidative stress in acute experimental colitis[J]. Antioxidants, 2023, 12(5):1062. doi: 10.3390/antiox12051062 [40] HU L Q, MAGESH S, CHEN L, et al. Discovery of a small-molecule inhibitor and cellular probe of Keap1-Nrf2 protein-protein interaction[J]. Bioorg Med Chem Lett, 2013, 23(10):3039-3043. doi: 10.1016/j.bmcl.2013.03.013 [41] ONTORIA J M, BIANCOFIORE I, FEZZARDI P, et al. Combined peptide and small-molecule approach toward nonacidic THIQ inhibitors of the KEAP1/NRF2 interaction[J]. ACS Med Chem Lett, 2020, 11(5):740-746. doi: 10.1021/acsmedchemlett.9b00594 [42] WEN X, THORNE G, HU L Q, et al. Activation of NRF2 signaling in HEK293 cells by a first-in-class direct KEAP1-NRF2 inhibitor[J]. J Biochem Mol Toxicol, 2015, 29(6):261-266. doi: 10.1002/jbt.21693 [43] TONG K I, KATOH Y, KUSUNOKI H, et al. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: characterization of the two-site molecular recognition model[J]. Mol Cell Biol, 2006, 26(8):2887-2900. doi: 10.1128/MCB.26.8.2887-2900.2006 [44] DAVIES T G, WIXTED W E, COYLE J E, et al. Monoacidic inhibitors of the kelch-like ECH-associated protein 1: nuclear factor erythroid 2-related factor 2(KEAP1: NRF2)protein-protein interaction with high cell potency identified by fragment-based discovery[J]. J Med Chem, 2016, 59(8):3991-4006. doi: 10.1021/acs.jmedchem.6b00228 [45] KASEDA S, SANNOMIYA Y, HORIZONO J, et al. Novel Keap1-Nrf2 protein-protein interaction inhibitor UBE-1099 ameliorates progressive phenotype in alport syndrome mouse model[J]. Kidney360, 2022, 3(4):687-699. doi: 10.34067/KID.0004572021 [46] BERTRAND H C, SCHAAP M, BAIRD L, et al. Design, synthesis, and evaluation of triazole derivatives that induce Nrf2 dependent gene products and inhibit the Keap1-Nrf2 protein-protein interaction[J]. J Med Chem, 2015, 58(18):7186-7194. doi: 10.1021/acs.jmedchem.5b00602 [47] LAO Y Q, WANG Y, CHEN J W, et al. Synthesis and biological evaluation of 1, 2, 4-triazole derivatives as potential Nrf2 activators for the treatment of cerebral ischemic injury[J]. Eur J Med Chem, 2022, 236:114315. doi: 10.1016/j.ejmech.2022.114315 [48] COSIMELLI B, GRECO G, LANERI S, et al. Identification of novel indole derivatives acting as inhibitors of the Keap1-Nrf2 interaction[J]. J Enzyme Inhib Med Chem, 2019, 34(1):1152-1157. doi: 10.1080/14756366.2019.1623209 [49] HERRERA-AROZAMENA C, ESTRADA-VALENCIA M, PÉREZ C, et al. Tuning melatonin receptor subtype selectivity in oxadiazolone-based analogues: Discovery of QR2 ligands and NRF2 activators with neurogenic properties[J]. Eur J Med Chem, 2020, 190:112090. doi: 10.1016/j.ejmech.2020.112090 [50] ZHOU H S, HU L B, ZHANG H, et al. Design, synthesis, and structure-activity relationships of indoline-based kelch-like ECH-associated protein 1-nuclear factor(erythroid-derived 2)-like 2(Keap1-Nrf2)protein-protein interaction inhibitors[J]. J Med Chem, 2020, 63(19):11149-11168. doi: 10.1021/acs.jmedchem.0c01116 [51] ZHOU H S, WANG Y, YOU Q D, et al. Recent progress in the development of small molecule Nrf2 activators: a patent review(2017-present)[J]. Expert Opin Ther Pat, 2020, 30(3):209-225. doi: 10.1080/13543776.2020.1715365 [52] ZHANG ZX, TIAN SW, YOU Y. The pathophysiological role of neuroinflammation in neurodegenerative and psychiatric disorders[J]. 中南医学科学杂志, 2017, 45(3):312-314. [53] YANG Y Y, LI X Y, PENG L Y, et al. Tanshindiol C inhibits oxidized low-density lipoprotein induced macrophage foam cell formation via a peroxiredoxin 1 dependent pathway[J]. Biochim Biophys Acta Mol Basis Dis, 2018, 1864(3):882-890. doi: 10.1016/j.bbadis.2017.12.033 [54] JAKOBS P, SERBULEA V, LEITINGER N, et al. Nuclear factor(erythroid-derived 2)-like 2 and thioredoxin-1 in atherosclerosis and ischemia/reperfusion injury in the heart[J]. Antioxid Redox Signal, 2017, 26(12):630-644. doi: 10.1089/ars.2016.6795 [55] CLERICI S, BOLETTA A. Role of the KEAP1-NRF2 axis in renal cell carcinoma[J]. Cancers, 2020, 12(11):3458. doi: 10.3390/cancers12113458 [56] TORRES V E, CHAPMAN A B, DEVUYST O, et al. Tolvaptan in later-stage autosomal dominant polycystic kidney disease[J]. N Engl J Med, 2017, 377(20):1930-1942. doi: 10.1056/NEJMoa1710030 [57] WU K C, LIU J, KLAASSEN C D. Role of Nrf2 in preventing ethanol-induced oxidative stress and lipid accumulation[J]. Toxicol Appl Pharmacol, 2012, 262(3):321-329. doi: 10.1016/j.taap.2012.05.010 [58] LIU WJ, CHEN WW, CHEN JY, et al. Baicalin attenuated metabolic dysfunction-associated fatty liver disease by suppressing oxidative stress and inflammation via the p62-Keap1-Nrf2 signalling pathway in db/db mice [J]. Phytother Res, 2023: 1-16. -




 下载:
下载: