-
黑色素瘤是一种源自皮肤色素细胞(黑色素细胞)的恶性肿瘤,其恶性程度高、转移速度快,是导致皮肤癌相关死亡的主要原因之一[1]。近年来,随着免疫治疗和靶向治疗的发展,黑色素瘤的治疗方法逐渐取得了显著进展。免疫检查点抑制剂如曲美布单抗(TREM)和靶向治疗药物如达拉非尼(DAB)已成为黑色素瘤治疗的主要选择之一[2-4]。然而,尽管这些治疗方法取得了较好的临床效果,仍然存在部分患者耐药或治疗反应不佳的情况,使得联合治疗成为一种有前景的治疗策略[5]。
TREM是一种抗CTLA-4单克隆抗体,通过解除对T细胞的抑制,增强免疫系统对肿瘤细胞的识别与清除作用[6]。DAB则是一种针对B-型RAF蛋白激酶(BRAF)突变的靶向药物,通过抑制BRAF突变引发的细胞增殖信号,从而抑制黑色素瘤细胞的增殖[7]。近年来的研究表明,免疫治疗与靶向治疗的联合使用能够通过相互作用提高治疗效果,并可能克服单一疗法的局限性[8-9]。然而,联合治疗是否能够有效提高黑色素瘤治疗效果及相关作用机制,尚未完全明确。
氧化应激在肿瘤的发生发展中发挥着重要作用[10-11]。肿瘤细胞通过调整其氧化还原状态来适应恶劣的微环境,并促进其生长、迁移和耐药性[12-14]。研究表明,黑色素瘤细胞常通过产生过量的活性氧(ROS)来推动其增殖及转移,而抑制这些ROS的生成或增强抗氧化机制的作用,可能成为癌症治疗的新策略[15-17]。此外,三磷酸腺苷(ATP)作为细胞的能量货币,对于维持细胞的基本功能和生存至关重要[18-19]。肿瘤细胞通常通过改变其能量代谢途径(如从有氧呼吸转向无氧糖酵解)来支持快速增殖[20-21]。因此,调控肿瘤细胞的能量代谢状态对于改善治疗效果和克服耐药性具有潜在意义[22]。
尽管已有研究探讨了单一药物(达拉非尼或曲美布单抗)在黑色素瘤中的疗效,但两者联合在黑色素瘤治疗中的作用及相关机制研究有限。因此,本研究旨在通过体内外实验,探索达拉非尼联合曲美布单抗通过调控氧化应激反应和能量代谢提高黑色素瘤的治疗效果。
-
DAB(批号:HY-14660)、TREM(批号:HY-B0380)(美国MCE公司);胎牛血清(FBS)(美国Gibco公司);胰蛋白酶、青霉素-链霉素(上海索莱宝公司);CCK-8、ROS、SOD和MDA检测试剂盒(上海碧云天生物科技公司);乳酸脱氢酶(LDH)、ATP检测试剂盒(南京建成生物科技公司);抗PCNA的抗体(武汉三鹰生物科技公司);荧光过氧化物酶 FITC 标记的二抗(美国Thermo Fisher Scientific公司);酶标仪(型号:Multiskan skyHigh,Thermo Fisherg公司);倒置显微镜(型号:DMi1,Leica公司);分光光度计(型号:Genesys 10S UV-Vis,Thermo Scientific公司)。
-
A375细胞(具有BRAF突变的黑色素瘤细胞系,上海富衡生物科技有限公司);BALB/c Nude雄性裸鼠,4~5周龄[实验动物生产许可证号:SYXK(渝)2024-0001,重庆吉立辉生物科技有限公司]。
-
选择A375细胞作为研究对象,使用含有10% FBS和1%青霉素-链霉素的DMEM培养基培养,并置于37℃、5% CO2的恒温培养箱中。每隔2 d更换一次培养液,待细胞生长至 80%~90%时,使用0.25%胰蛋白酶进行传代。
-
采用CCK-8法测定DAB联合TREM对A375细胞生长活力的影响。取对数生长期的A375细胞,以1.0×104个/孔接种至96孔板中。培养24 h后,分别加入DAB(1 μmol/L)、TREM(5 μg/ml)和DAB(1 μmol/L)+TREM(5 μg/ml),同时设置空白组和溶剂对照组(0.1% DMSO)。药物处理24 h后,将原培养基换成新鲜培养基,每孔加入10 μl CCK-8溶液,继续孵育1 h。孵育结束后,使用酶标仪在450 nm处测定各组的吸光度(A值),并计算细胞存活率:细胞存活率(%)=(实验组平均A值–空白组平均A值)/(溶剂对照组平均A值–空白组平均A值)。
-
采用测定LDH活性法评估药物对A375细胞的细胞毒性作用。细胞分组及处理过程同1.4项,药物处理后,收集每孔的培养基,加入LDH反应液,孵育1 h后,使用酶标仪于490 nm处测定A值。根据A值计算细胞死亡率,使用溶剂对照组和最大释放组作为参考,最终得出不同药物处理组的细胞毒性情况。
-
将对数生长期的A375细胞(1.0×105个/孔)接种于6孔板中,待细胞贴壁生长至整个底部时,使用200 μl无菌移液枪枪头在板表面划出一条等宽的线,用PBS洗涤2次去除划痕区域的细胞碎片。分别加入DAB(1 μmol/L)、TREM(5 μg/ml)和DAB(1 μmol/L)+TREM(5 μg/ml),并设置溶剂对照组,继续培养24 h。在划痕后0、24 h于倒置显微镜下观察并拍摄划痕区域,使用Image J软件定量分析划痕宽度的变化,并计算划痕愈合率:划痕愈合率(%)=(0 h划痕宽度–24 h划痕宽度)/0 h划痕宽度×100%。
-
取对数生长期的A375细胞,以1.0×104个/孔接种至96孔板中,培养24 h。分别加入DAB(1 μmol/L)、TREM(5 μg/ml)和DAB(1 μmol/L)+TREM(5 μg/ml)进行处理,并设置溶剂对照组。药物处理2 h后,加入DCFH-DA探针溶液(10 μmol/L),孵育30~60 min,使细胞摄取并与细胞内的ROS反应,生成具有荧光特性的DCF产物。用PBS溶液洗涤3次后,加入适量PBS,使用酶标仪在530 nm波长处测定每孔的荧光强度。
-
取对数生长期的A375细胞,以1.0×105个/孔接种至6孔板中,培养24 h。分别加入DAB(1 μmol/L)、TREM(5 μmol/L)和DAB(1 μmol/L)+TREM(5 μg/ml)进行处理,并设置溶剂对照组。药物处理24 h后,使用细胞裂解液将细胞裂解。
收集各组处理后的A375细胞裂解液,并转移至96孔板中,加入适量的ATP检测试剂,反应完成后,通过酶标仪测定每孔的吸光度值,并计算各组样品中ATP水平。
分别取50 μl各组处理后的A375细胞裂解液到新的离心管中,加入适量的硫代巴比妥酸(TBA)试剂。将试剂和样本混合后,放入95℃的水浴中加热0.5 h,在此过程中,MDA与TBA反应生成红色的复合物。反应结束后,将样本从水浴中取出,冷却至室温,10 000 r/min 离心5 min,去除可能存在的沉淀。最后,使用分光光度计在532 nm处测定样本的吸光度,根据标准曲线计算样品中MDA水平。
收集各组处理后的A375细胞裂解液,并转移至96孔板中,加入适量的SOD底物溶液。反应完成后,使用酶标仪在450 nm处测定每孔的吸光度,计算各组样品中SOD水平。
-
收集对数生长期的A375细胞,用PBS重悬细胞,加入等量Matrigel基质胶,调整细胞密度(1.0×107个/ml),混匀后置于冰上。将100 μl细胞悬液注射于裸鼠右侧腋窝皮下,1周后,在裸鼠皮下可见实体瘤形成。当肿瘤长至100 mm3(肿瘤体积=1/2×长径×短径2)时,将裸鼠随机分为DAB (30 mg/kg)组、TREM(10 μg/kg)组、DAB(30 mg/kg)+TREM(10 μg/kg)组和溶剂对照组(含5% DMSO及20% PEG400的生理盐水),每组6只,每2 d尾静脉注射给药1次,共给药21 d。21 d后脱颈处死裸鼠,剥离肿瘤组织并称量肿瘤质量。
-
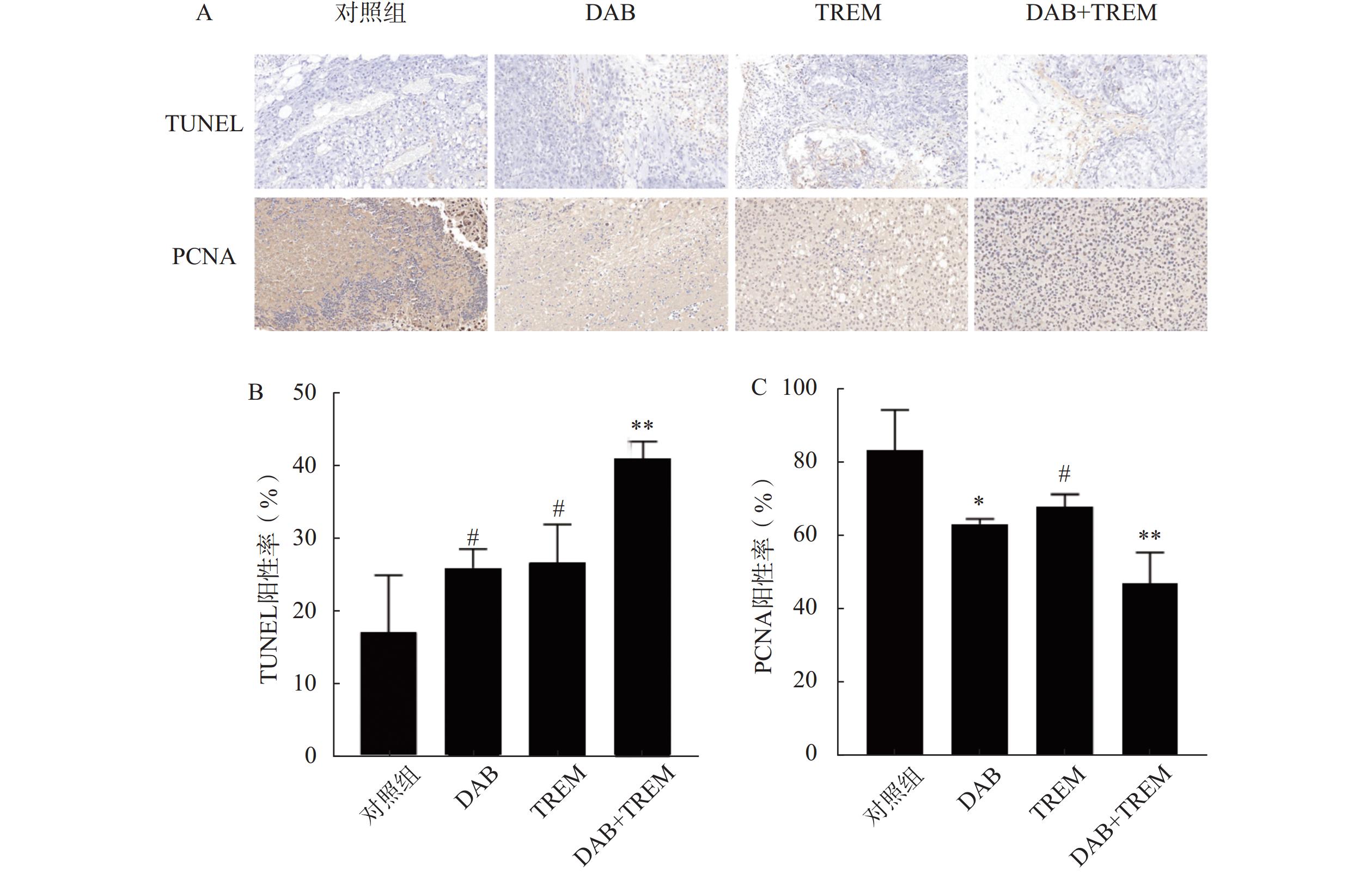
将剥离的肿瘤组织经4%多聚甲醛固定24 h后,进行石蜡包埋,切片厚度为4 μm。切片经二甲苯脱蜡 2 次,每次 5~10 min,依次经无水乙醇、90%乙醇、70%乙醇及蒸馏水梯度水化。滴加 20 μg/ml无 DNA 酶污染的蛋白酶K,37℃孵育 20 min 进行抗原修复,PBS冲洗3次。对于TUNEL实验,使用TUNEL试剂盒按照说明书操作。具体步骤为:切片经过PBS洗涤后,加入TUNEL反应试剂,于37℃孵育60 min,加入核染色剂后继续孵育5 min。染色后,使用显微镜观察细胞凋亡信号的标记,TUNEL阳性细胞核呈棕黄色,非凋亡细胞核呈蓝色,并计算TUNEL阳性率:阳性率(%)=阳性细胞数/总细胞数×100%,以定量评估细胞凋亡。对于PCNA免疫组化,切片经过脱蜡、水合后,采用3%过氧化氢封闭内源性过氧化物酶,随后加入针对PCNA的特异性一抗,4℃孵育过夜。次日,加入二抗,室温下孵育1 h,最后使用DAB显色试剂盒显色,并用苏木素复染。切片脱水后封片,通过显微镜观察PCNA阳性细胞(细胞核出现棕黄色颗粒沉着)的分布与表达,并计算PCNA阳性率:阳性率(%)=阳性细胞数/总细胞数×100%,以定量评估细胞增殖。
-
数据统计和分析均采用 SPSS 26.0和GraphPad Prism 8.0 软件进行,数据以(
$\bar x $ ±s)表示。采用Student’s t test 和单因素方差分析进行组间差异的比较,P<0.05说明差异具有统计学意义。 -
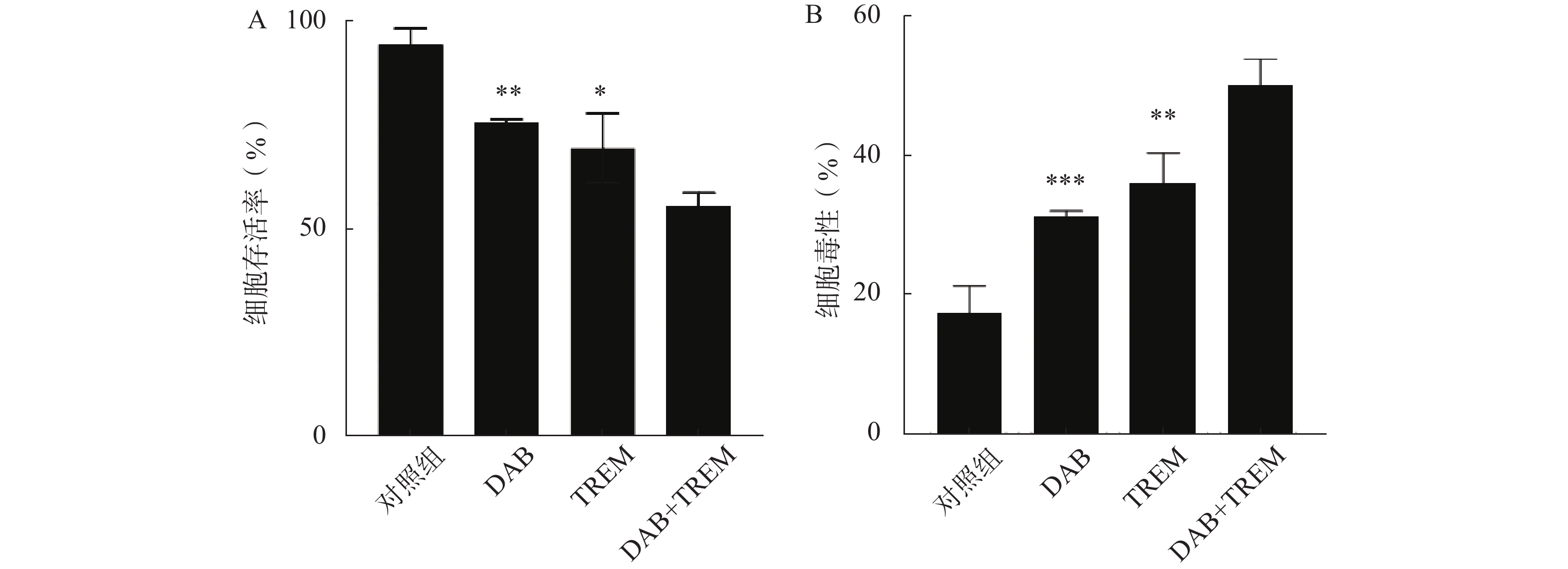
采用CCK8法检测单独给药及DAB联合TREM对A375细胞生长活力的影响。结果如图1A所示,与对照组相比,单独给药组及联合给药组的细胞存活率均显著降低,提示DAB和TREM对A375细胞均具有细胞增殖抑制作用;且与单药组相比,联合给药组的细胞存活率显著降低,分别为(75.76±0.79)%、(69.64±8.34)%和(55.88±3.01)%,提示这两种药物可能通过协同作用增强治疗效果。
为了进一步检测各组对A375细胞的毒性作用,采用测定生长介质中由受损细胞释放的LDH活性的方法。结果如图1B所示,与对照组相比,单独给药组及联合给药组的细胞毒性均显著提高,表明这两种单药对A375细胞具有一定的毒性作用;DAB联合TREM组的细胞毒性显著高于单独给药组,分别为(31.24±0.82)%、(35.93±4.43)%和(50.00±3.83)%,提示联合给药对A375细胞的毒性效果更为显著。
-
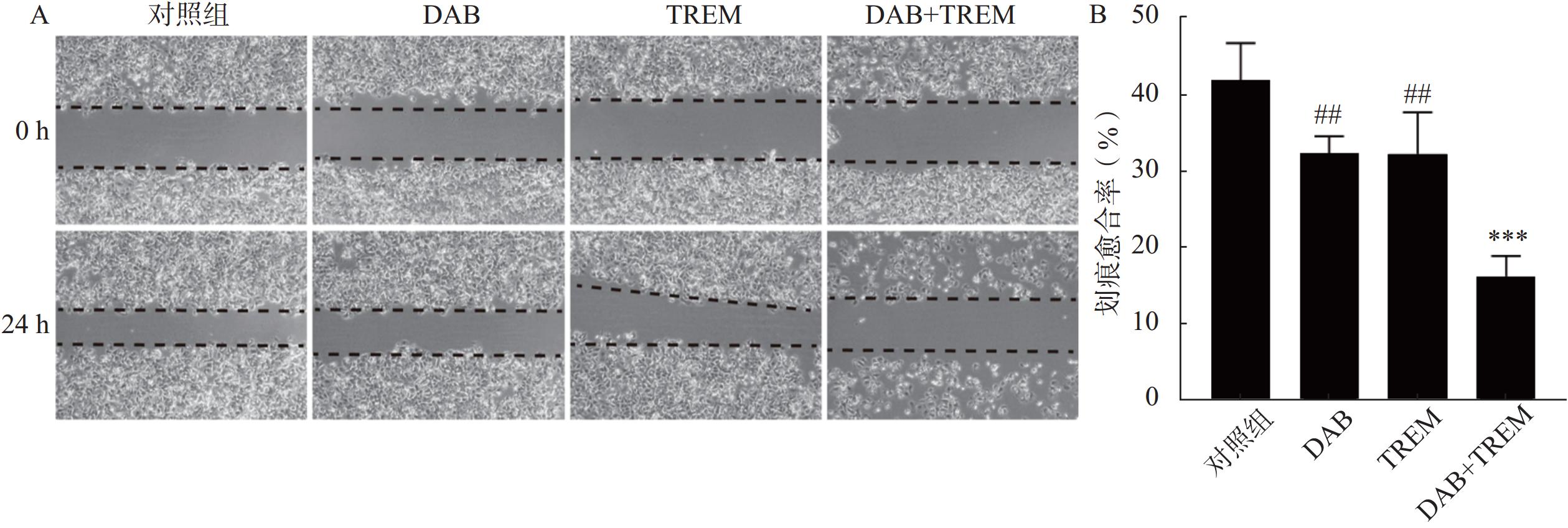
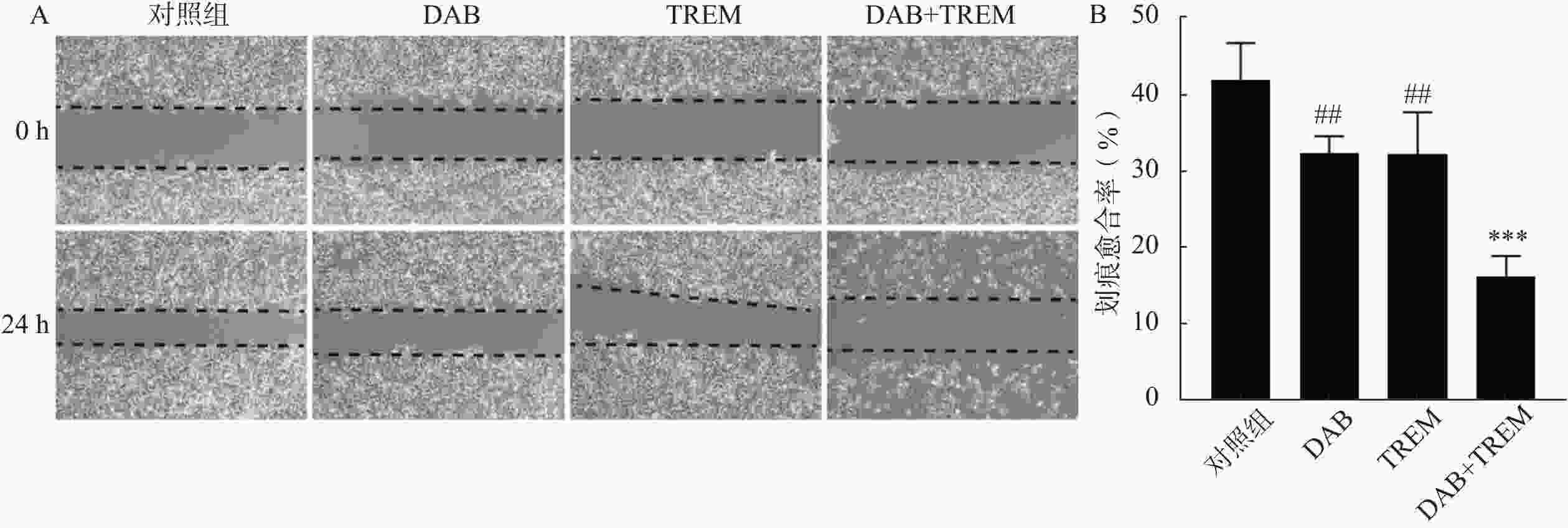
为了评估各组对A375细胞迁移能力及细胞愈合抑制作用,进行了细胞划痕实验。结果如图2A所示,0 h时所有组的A375细胞表面均可观察到明显的划痕,且细胞状态差异不大。经过24 h处理后,对照组的细胞成功愈合了大部分划痕,愈合速度较快。相比之下,DAB、TREM和DAB联合TREM组的细胞愈合速度较慢,其中,联合给药组的愈合速度最慢,表现出明显的愈合抑制作用。通过量化划痕愈合率,如图2B所示,与对照组相比,单独给药组未显示显著性差异,愈合率分别为(41.95±4.76)%、(32.35±2.22)%和(32.28±5.40)%;联合给药组的愈合率为(16.25±2.68)%,与其他组相比显著降低,提示联合给药在细胞迁移和愈合过程中产生了较强的抑制作用。
-
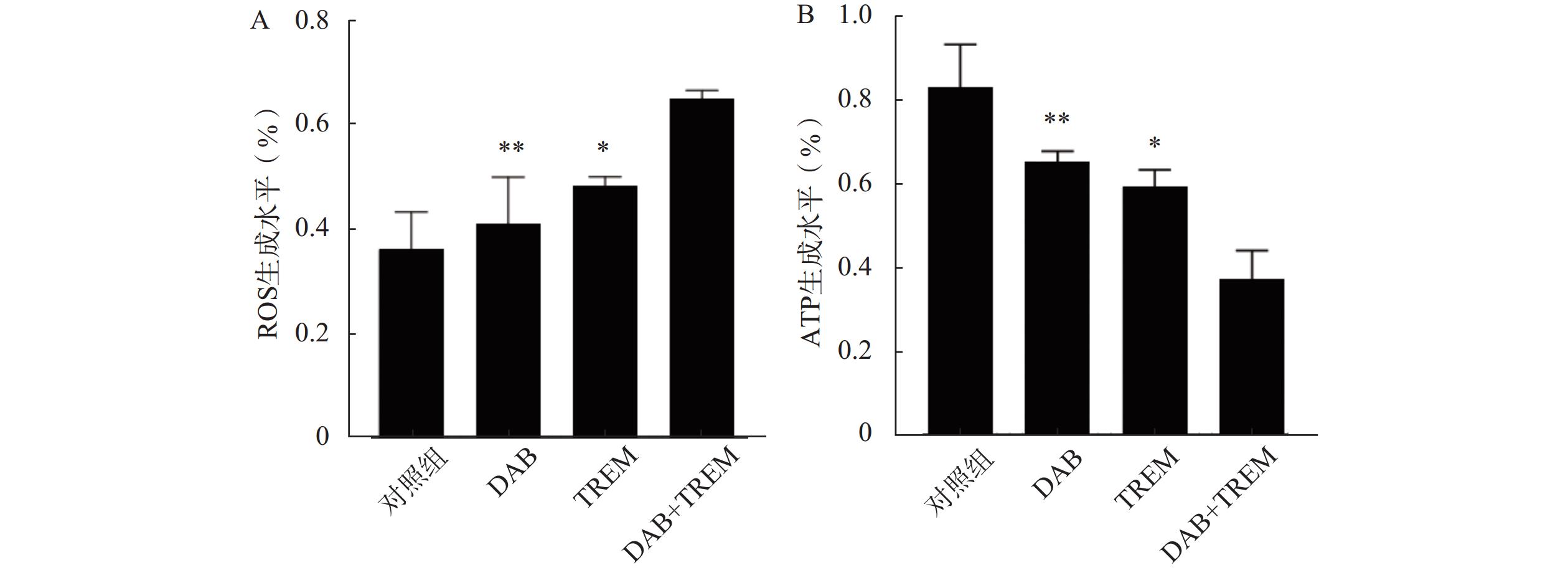
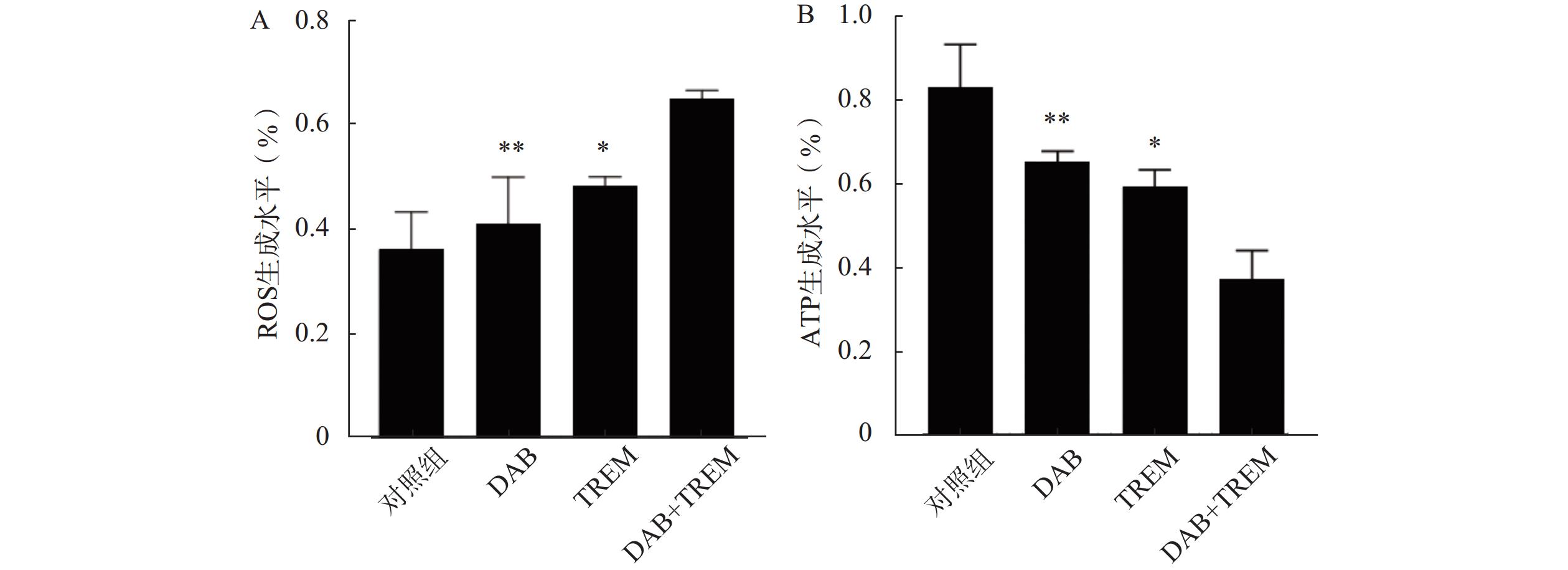
结果如图3A所示,与对照组相比,单独给药组的ROS水平有所增加,但差异不显著;而DAB联合TREM组的ROS水平较对照组及单独给药组显著增高,提示联合用药可能通过促进肿瘤细胞内ROS的积累及氧化应激反应而增强抗肿瘤作用。
如图3B所示,与对照组相比,单独给药组及联合给药组的ATP水平均显著降低,且联合用药组的ATP水平显著低于单独给药组。结果表明,DAB联合TREM可能通过抑制细胞的能量代谢而增强抗肿瘤作用。
-
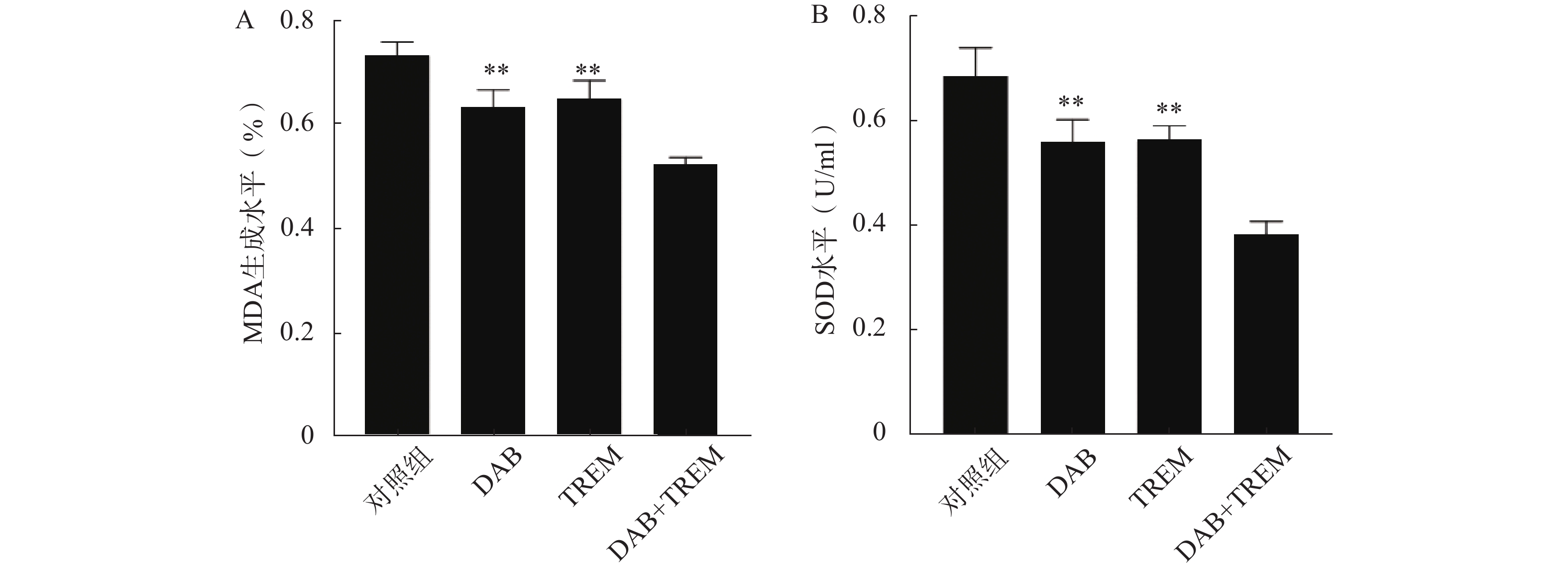
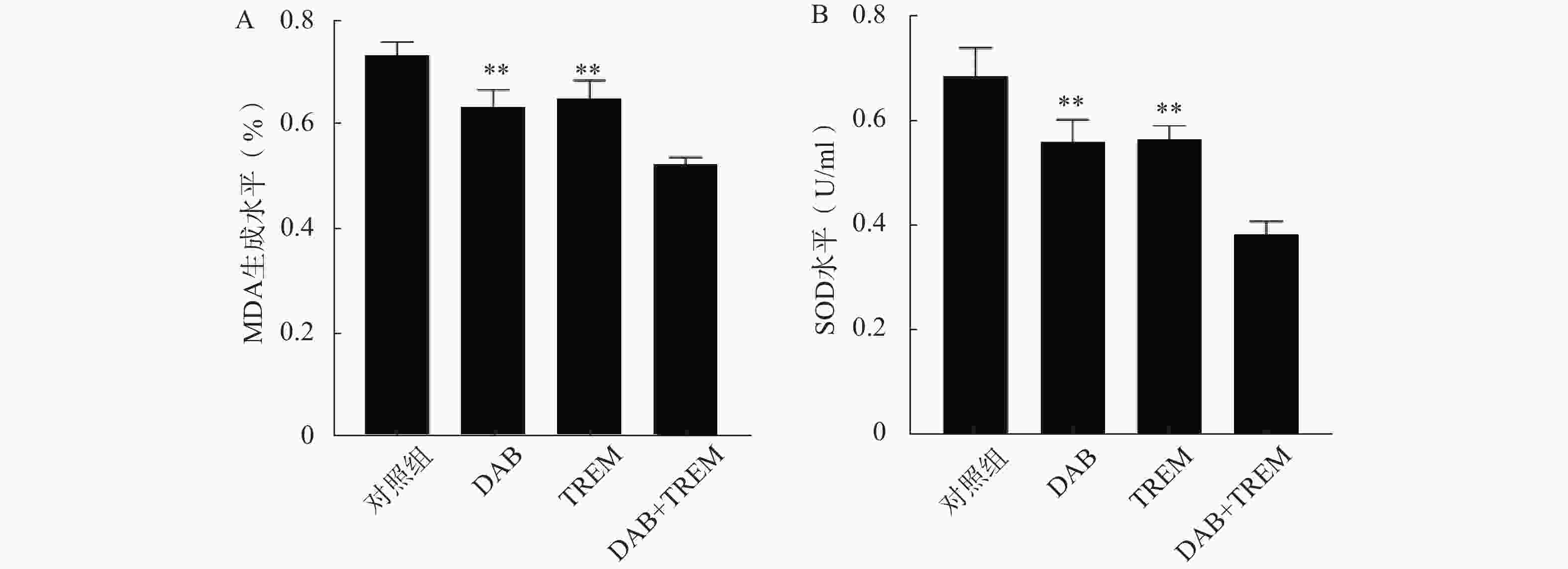
MDA含量测定可评价脂质过氧化水平,SOD活性测定反映细胞抗氧化能力。如图4所示,在MDA和SOD水平测定中,单独给药组及联合给药组的MDA和SOD水平较对照组均显著降低;且联合给药组的MDA和SOD水平显著低于单独给药组,提示DAB联合TREM可能通过增强氧化应激反应,降低细胞抗氧化能力,从而增强抗肿瘤效果。
-
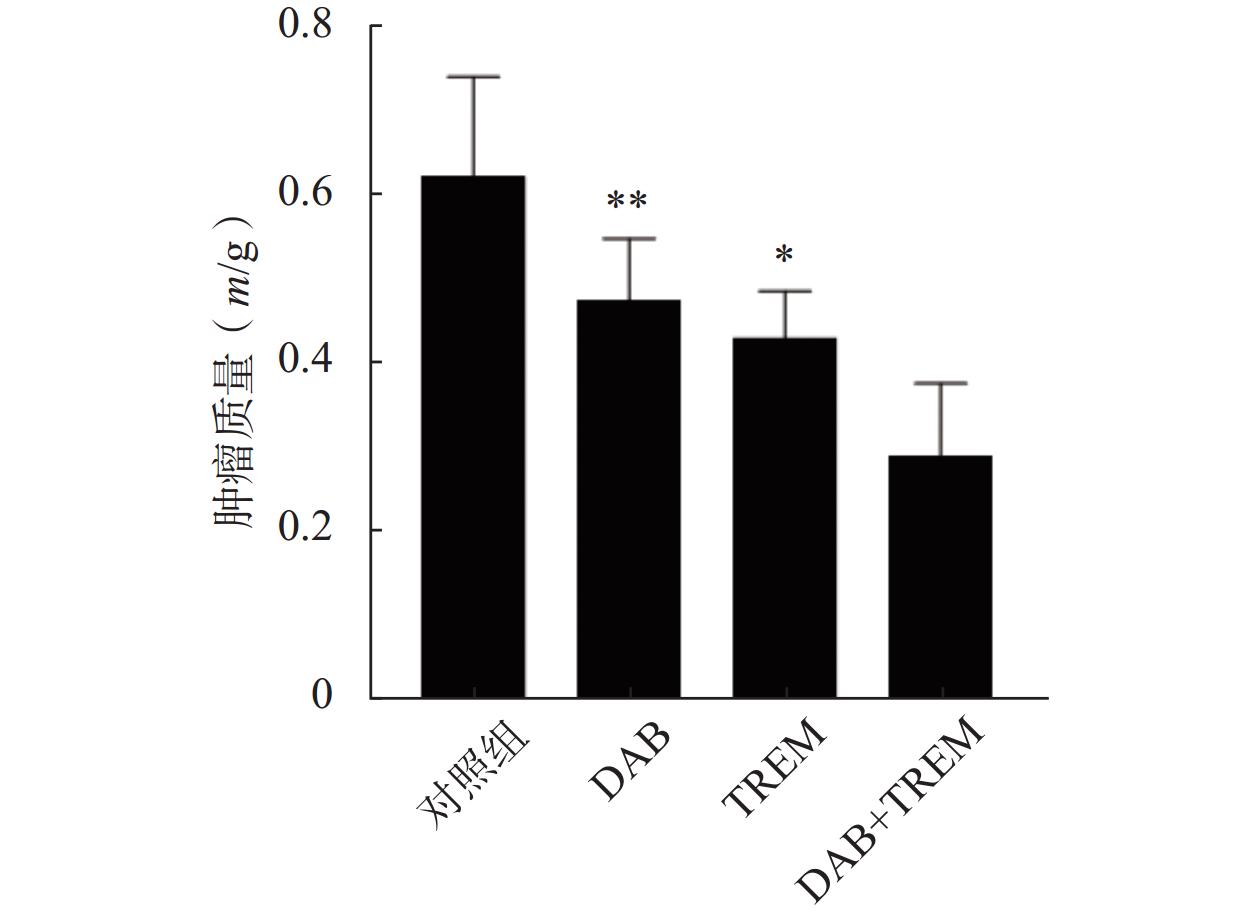
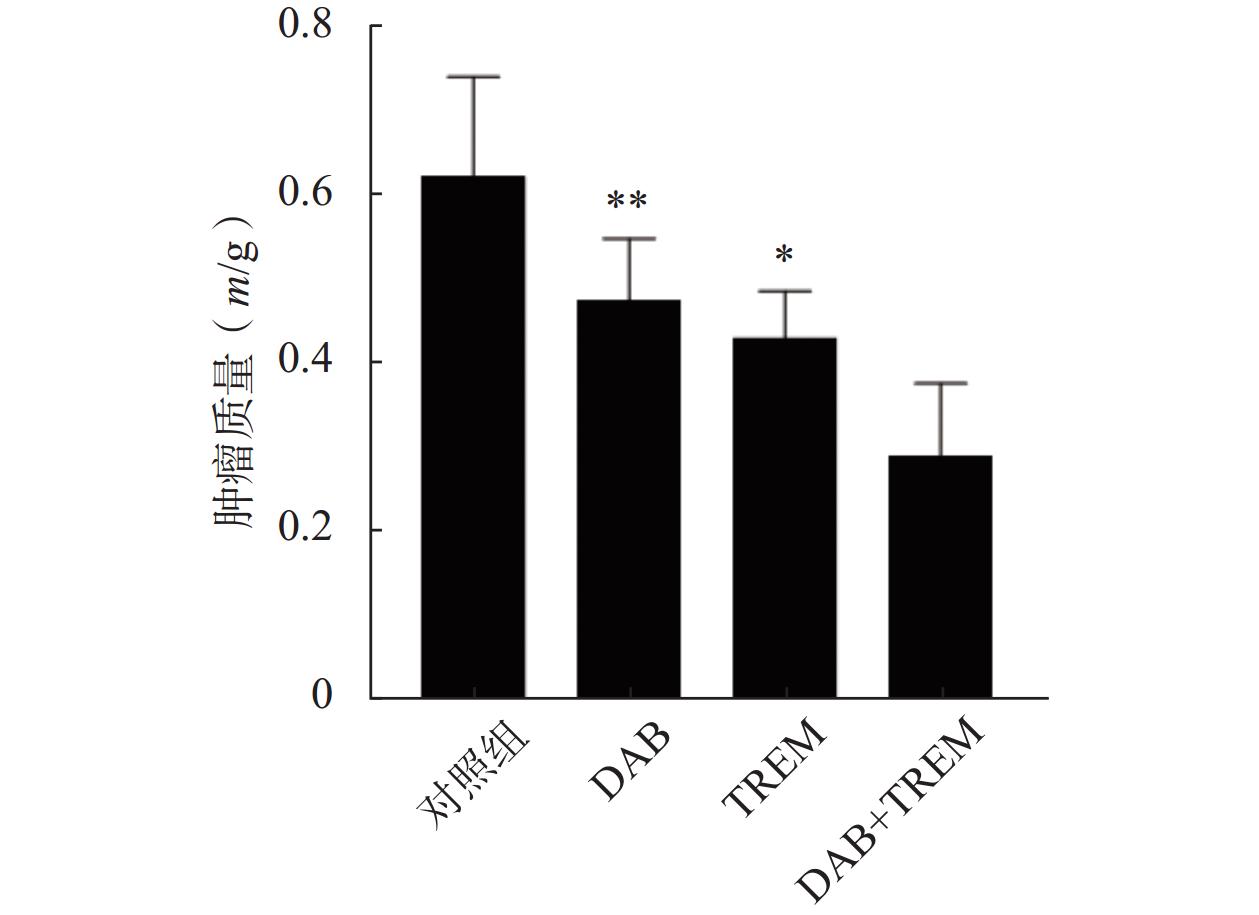
通过在裸鼠皮下注射A375黑色素瘤细胞建立了皮下移植瘤模型。结果如图5所示,与对照组相比,单独给药组及DAB联合TREM组均显著抑制了肿瘤生长,肿瘤质量分别为(0.62±0.12) g、(0.47±0.07) g、(0.43±0.06) g和(0.29±0.09) g;且联合给药组的肿瘤质量较单独给药组显著降低,证实DAB联合TREM能有效抑制体内黑色素瘤的生长,具有更强的抗肿瘤效果。
-
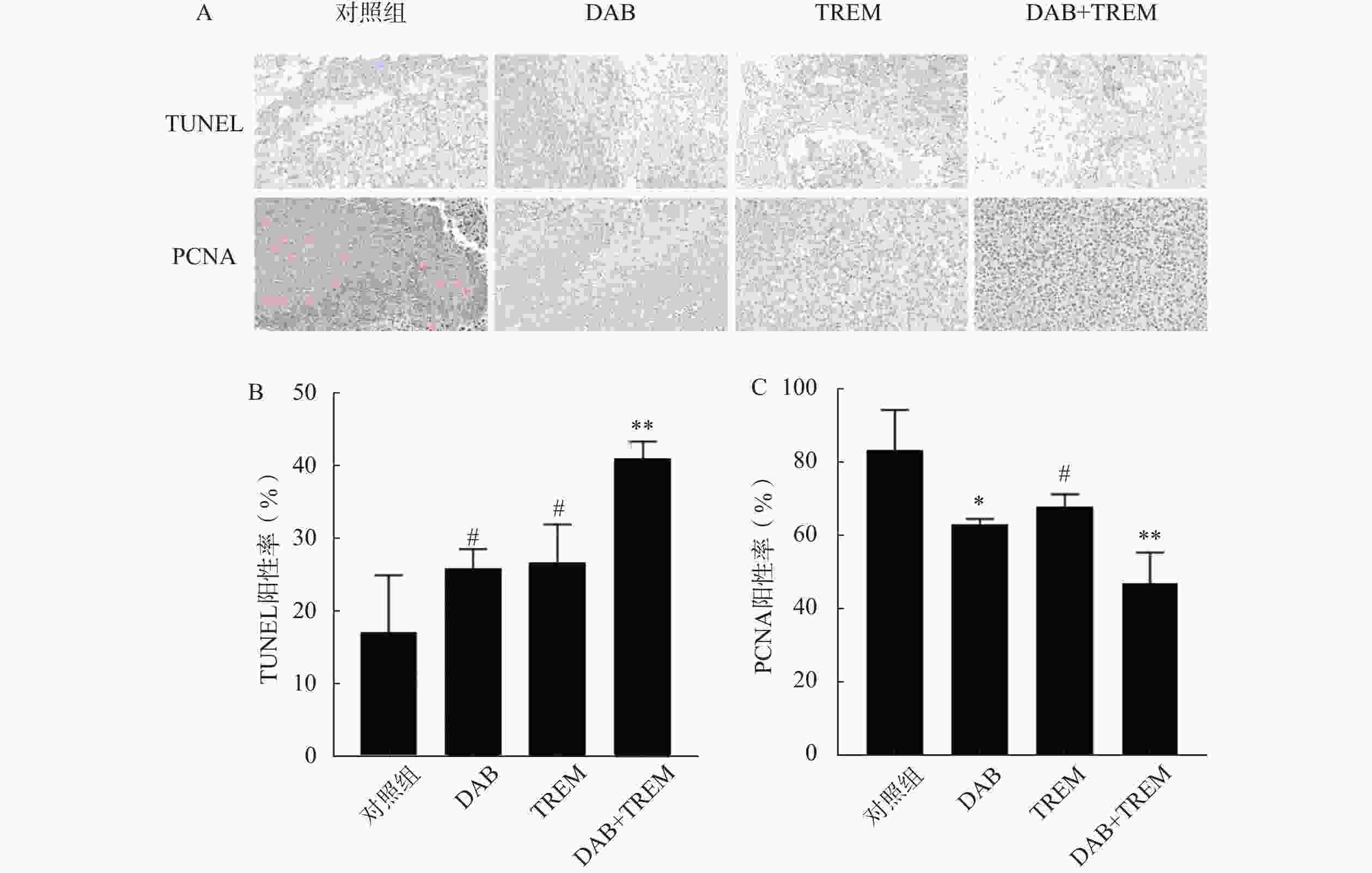
采用TUNEL凋亡染色法评价各组肿瘤组织细胞凋亡情况。结果如图6A和6B所示,与对照组相比,单独给药组的TUNEL阳性率有所提高,分别为(17.11±7.83)%、(25.94±2.59)%和(26.72±5.17)%,但差异并不显著;联合给药组的阳性率为(40.91±2.34)%,显著高于其他组,表明DAB与TREM联合应用时显著提高肿瘤细胞的凋亡率。
通过检测肿瘤组织内PCNA蛋白的表达,评价各组肿瘤组织细胞增殖情况。结果如图6A和6B所示,与对照组相比,单独给药组及联合给药组的PCNA阳性率均降低,分别为(83.091±11.00)%、(63.31±1.31)%、(68.00±3.25)%和(47.05±8.48)%。其中,联合给药组的PCNA阳性率降低最显著,提示DAB联合TREM显著抑制细胞增殖。综上,DAB联合TREM可能通过增强细胞凋亡及抑制细胞增殖而发挥更强的抗肿瘤效果。
-
黑色素瘤作为一种侵袭性极强的皮肤恶性肿瘤,严重威胁人类健康。目前单一治疗手段(如达拉非尼、曲美布单抗)存在疗效有限、易耐药等问题,而联合治疗可通过多靶点干预,成为黑色素瘤治疗研究的热点。
氧化应激与能量代谢在黑色素瘤发生、发展、转移及耐药过程中发挥关键作用。氧化应激是细胞内ROS产生与抗氧化防御失衡的状态。调节氧化应激的治疗策略主要有:①促氧化治疗:通过进一步升高肿瘤细胞内 ROS 水平,突破其耐受阈值,诱导细胞死亡。②抑制抗氧化防御:削弱黑色素瘤细胞的抗氧化能力,使其对氧化损伤更敏感。正常细胞主要依赖线粒体氧化磷酸化高效生成 ATP,满足生理需求。而黑色素瘤细胞则呈现出显著的ATP代谢重编程。调节能量代谢疗法的核心,在于精准干预黑色素瘤细胞异常的能量代谢模式,切断其“能量供应”,从而抑制肿瘤生长与扩散。调节能量代谢的治疗策略主要有靶向糖酵解途径、干预线粒体代谢和调控氨基酸与脂质代谢等途径。近年来,研究发现联合治疗可通过协同调节这两大生理过程,提高黑色素瘤治疗效果。
本研究表明,DAB联合TREM显著抑制黑色素瘤细胞活力与细胞迁移能力,并增强细胞毒性作用;显著提高ROS水平,降低MDA、SOD和ATP水平;有效抑制荷瘤裸鼠体内肿瘤生长,提高肿瘤组织细胞凋亡率且抑制细胞增殖,证实联合用药可能通过增强氧化应激、抑制能量代谢和促进细胞凋亡的作用机制提高黑色素瘤治疗效果。这些发现表明,DAB联合TREM能够克服单一药物的局限性,为黑色素瘤提供一种新的治疗策略。然而,未来的研究应进一步探索其分子作用机制,并评估其临床应用的安全性和长期疗效。
Effect and mechanism of dabrafenib combined with tremelimumab on melanoma
doi: 10.12206/j.issn.2097-2024.202504070
- Received Date: 2025-04-16
- Rev Recd Date: 2025-07-29
- Available Online: 2025-10-21
- Publish Date: 2025-10-25
-
Key words:
- dabrafenib /
- tremelimumab /
- melanoma /
- combination therapy /
- mechanism
Abstract:
| Citation: | WANG Xiaosong, LIU Yunjiao, ZHOU Jin, ZHANG Qianqian, MENG Lingjie. Effect and mechanism of dabrafenib combined with tremelimumab on melanoma[J]. Journal of Pharmaceutical Practice and Service, 2025, 43(10): 496-502. doi: 10.12206/j.issn.2097-2024.202504070 |








 DownLoad:
DownLoad: