-
稳定的清醒状态是生存和高级认知功能得以实现的前提。白天过度嗜睡(EDS)作为多种睡眠障碍和神经退行性疾病的常见症状,显著削弱认知功能[1-3]。睡眠剥夺是导致EDS和认知障碍的重要因素[4-5],现有药物莫达非尼有一定疗效但不排除成瘾风险[6]。组胺H3受体反向激动剂替洛利生通过增强组胺能传递有效改善EDS,安全性良好,但其可能的认知改善作用尚未被明确证实[7-14]。本研究采用小鼠睡眠剥夺模型,旨在验证替洛利生在促醒的同时是否也具备认知改善作用,为拓展其在睡眠障碍、阿尔茨海默病、帕金森病等神经退行性疾病治疗中的应用提供依据。
-
本研究采用12周龄、体质量25~30 g的C57BL/6J雄性小鼠。所有小鼠均接受脑电(EEG)与肌电(EMG)记录电极植入手术[8]。在2%异氟醚维持麻醉下,使用立体定位仪固定头部,于颅骨额叶与顶叶区域钻孔植入皮层电极以记录EEG信号,同时在颈部肌肉中植入EMG电极用于监测肌张力及运动状态。所有电极通过牙科黏合剂与牙科水泥固定。术后给予常规护理,确保小鼠正常进食、饮水和康复。
伦理声明:本研究严格遵守《中华人民共和国实验动物管理条例》及海军军医大学实验动物伦理委员会的指导原则,最大限度减少实验动物的数量与痛苦,所有实验方案获伦理批准。
-
术后恢复7 d后,小鼠转入隔音睡眠监测室进行环境适应。利用多导睡眠记录系统连接可旋转接头电缆,保证自由活动条件下连续监测EEG/EMG信号。第3周进行24 h基线睡眠-清醒周期记录,并量化分析清醒(W)、慢波睡眠(SWS)和异相睡眠(PS)所占比例及脑电频谱特征。使用Sleepsign软件以10 s为单位进行自动判读,结合人工校正以提高判别睡眠-清醒周期的准确性。
-
采用自动旋转杆系统实施不同持续时间的睡眠剥夺:6、12、24 h。剥夺期间小鼠可正常饮水与摄食,旋转杆以缓慢速度转动干扰其入睡。在剥夺结束前30 min,依据实验分组给予替洛利生(20 mg/kg)或生理盐水对照,之后继续记录至少6 h的睡眠-清醒状态。
-
用于评估小鼠在新环境中的自发活动及焦虑样行为。将小鼠放置于开放场中央,记录20 min内的总移动距离、中心与边缘区域停留时间、探索行为和竖立次数。每次测试后彻底清洁场地以避免留下气味干扰下一组动物。
-
用于检测小鼠的空间学习与记忆能力,实验包括5 d的隐藏平台训练和1 d的空间探索测试。训练阶段平台位于固定象限,小鼠从不同位置入水,限时60 s寻找平台,若未找到则引导其停留在平台上20 s。在测试阶段移除平台,小鼠在水池中自由游泳60 s,记录其穿越原平台位置的次数及在目标象限的停留时间,以评估其对空间位置的记忆保持能力。
-
所有实验数据以均值±标准误(Mean±SEM)表示,采用GraphPad Prism软件进行统计处理。组间差异通过双尾t检验或单因素方差分析(One-Way ANOVA)比较,P<0.05 视为具有统计学意义。
-
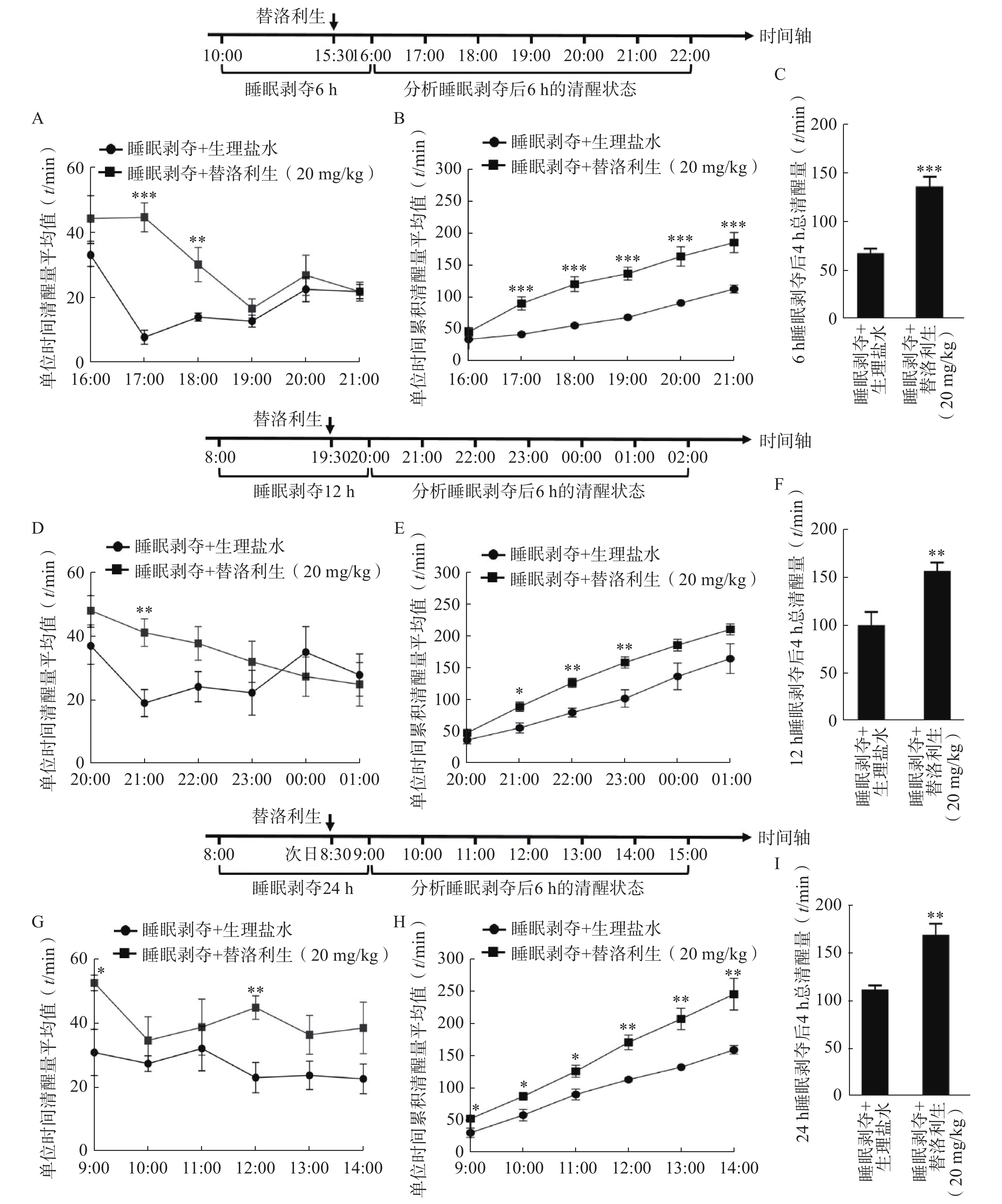
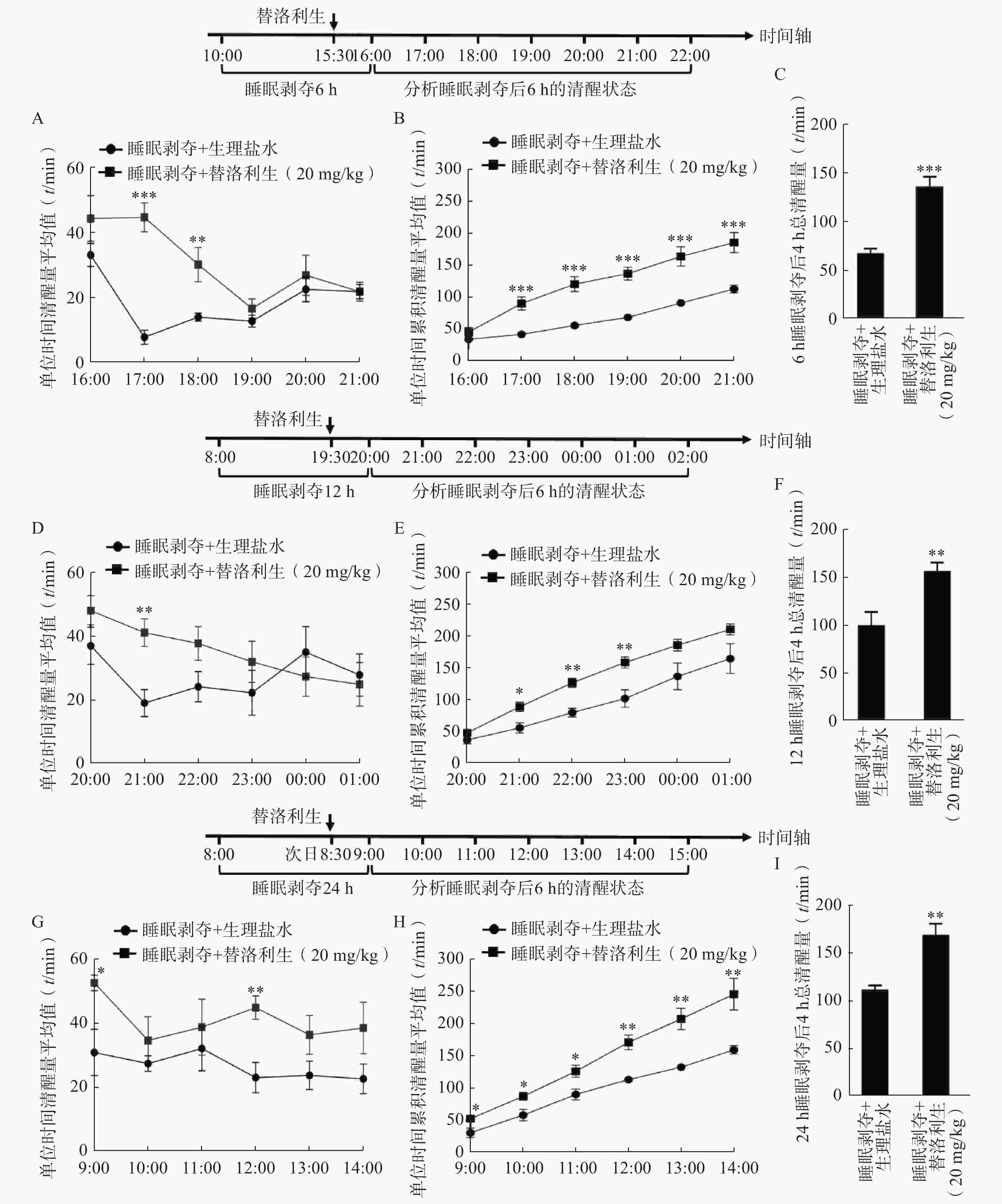
为评估替洛利生在不同睡眠剥夺时程后的促醒效果,分别在6、12和24 h的睡眠剥夺模型中,于剥夺结束前30 min给予口服替洛利生(20 mg/kg)[17]。
在6 h睡眠剥夺(10:00–16:00)模型中,替洛利生显著延长小鼠在后续6 h(16:00–22:00)内的清醒时间,尤其在给药后最初3 h内促醒效应最为明显。与对照组相比,替洛利生组小鼠的单位时间清醒量(图1A)、累积清醒量(图1B)及4 h总清醒时长(图1C)均显著提升(P<0.05)。其中,替洛利生组小鼠4 h总清醒时长[(136±10) min]比对照组[(67±5) min]增加了50% (n=10, P<0.001,图1C)。
12 h睡眠剥夺(08:00-20:00)模型中,替洛利生仍可有效提升清醒状态,在给药后前2 h内表现出显著的促醒优势(图1D~1F)。
在24 h睡眠剥夺(09:00–次日09:00)后,小鼠处于更高睡眠压力下,替洛利生依然能显著提高每小时清醒时间(图1G、1H),特别是在给药后4 h内,替洛利生组在总清醒时间方面[(171±11) min]显著高于对照组[(113±4) min],增加了34%(P=
0.0012 ,图1I),显示出其促醒效应的持续性与稳定性。综上,替洛利生在短期与长期睡眠剥夺后均表现出良好的促醒作用,且其作用在剥夺结束后早期阶段最为突出,提示其在睡眠障碍相关疾病,尤其嗜睡和过度睡眠中的潜在应用价值。
-
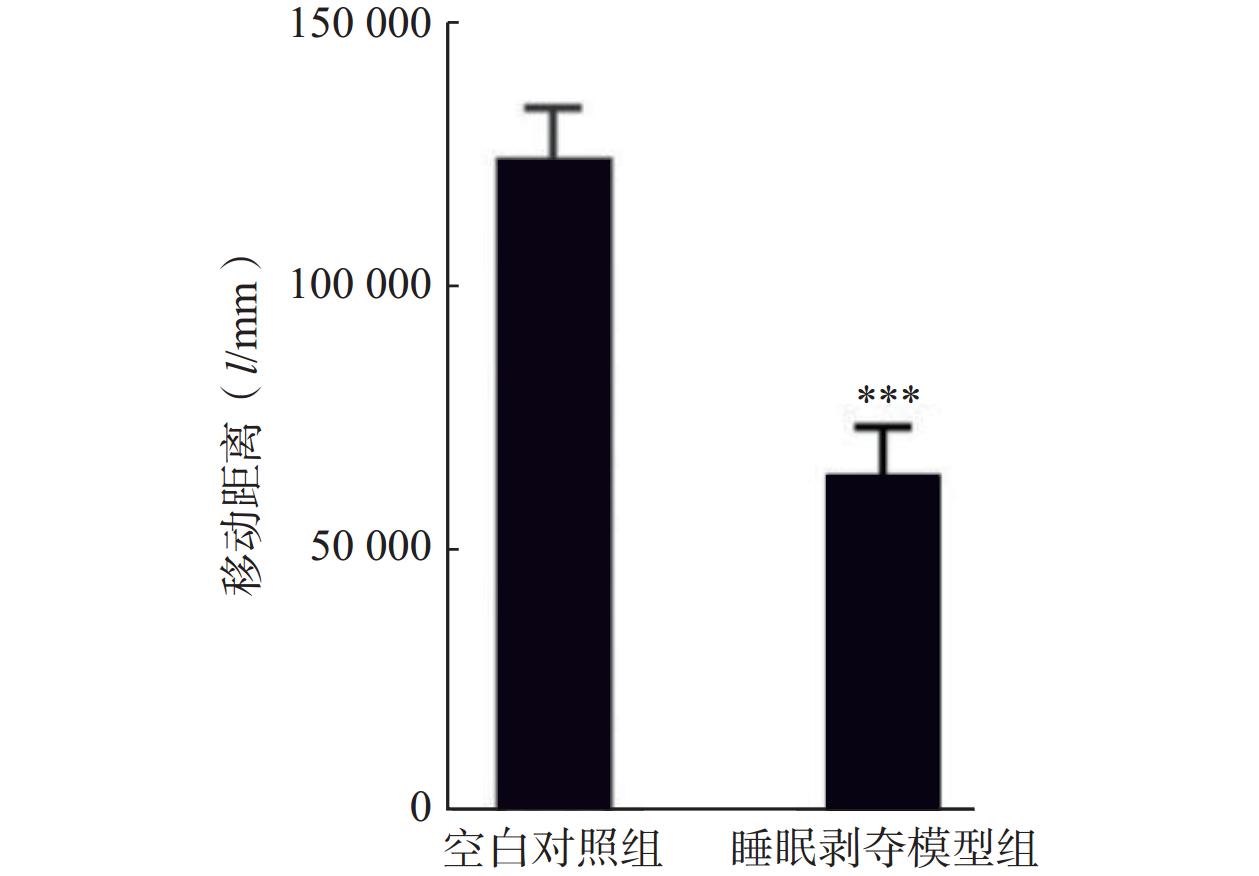
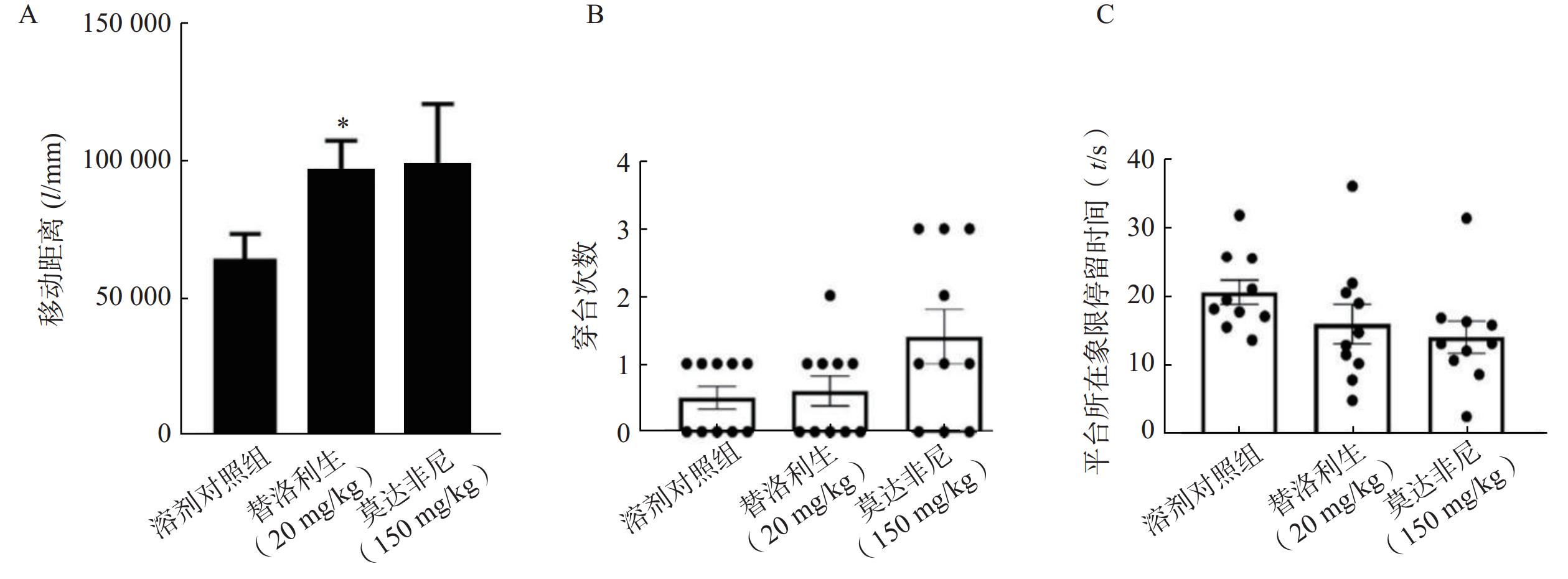
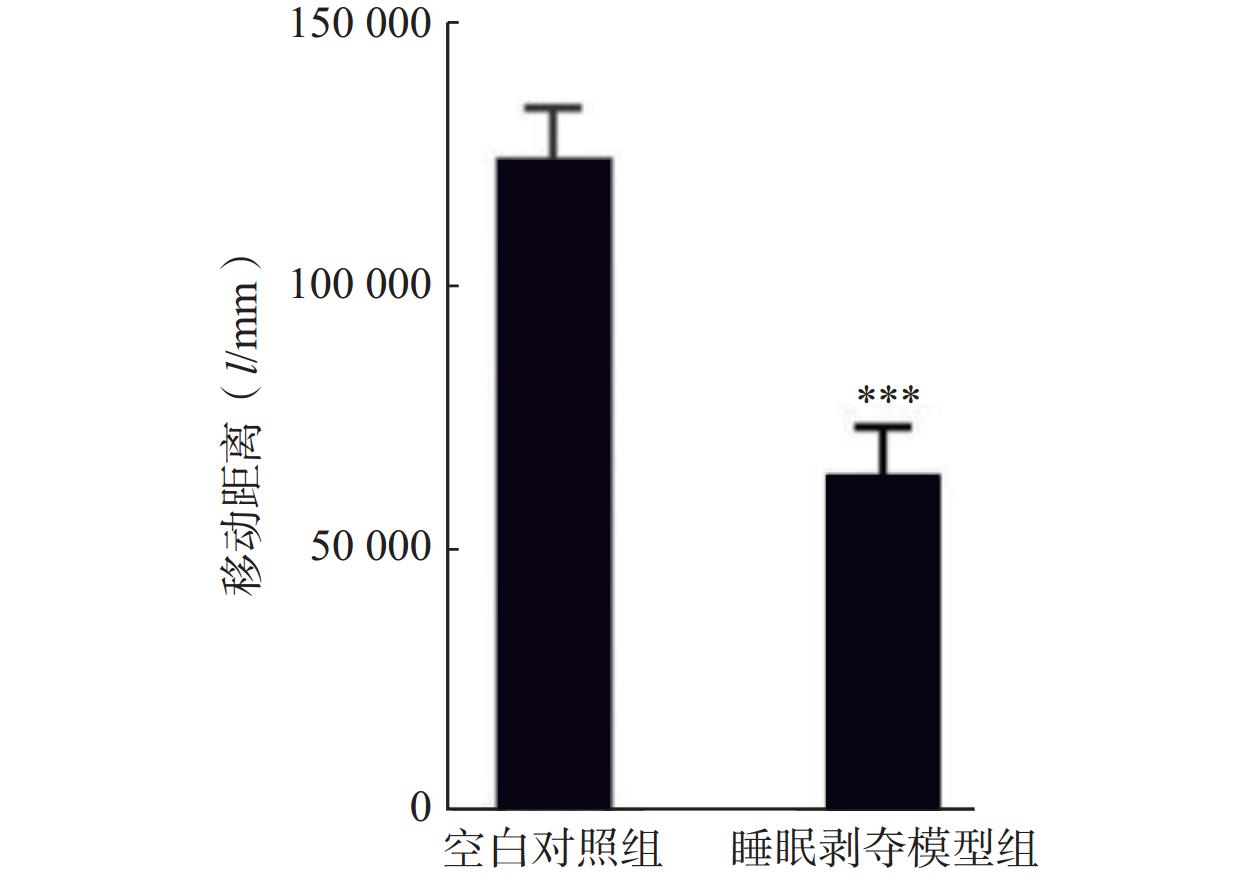
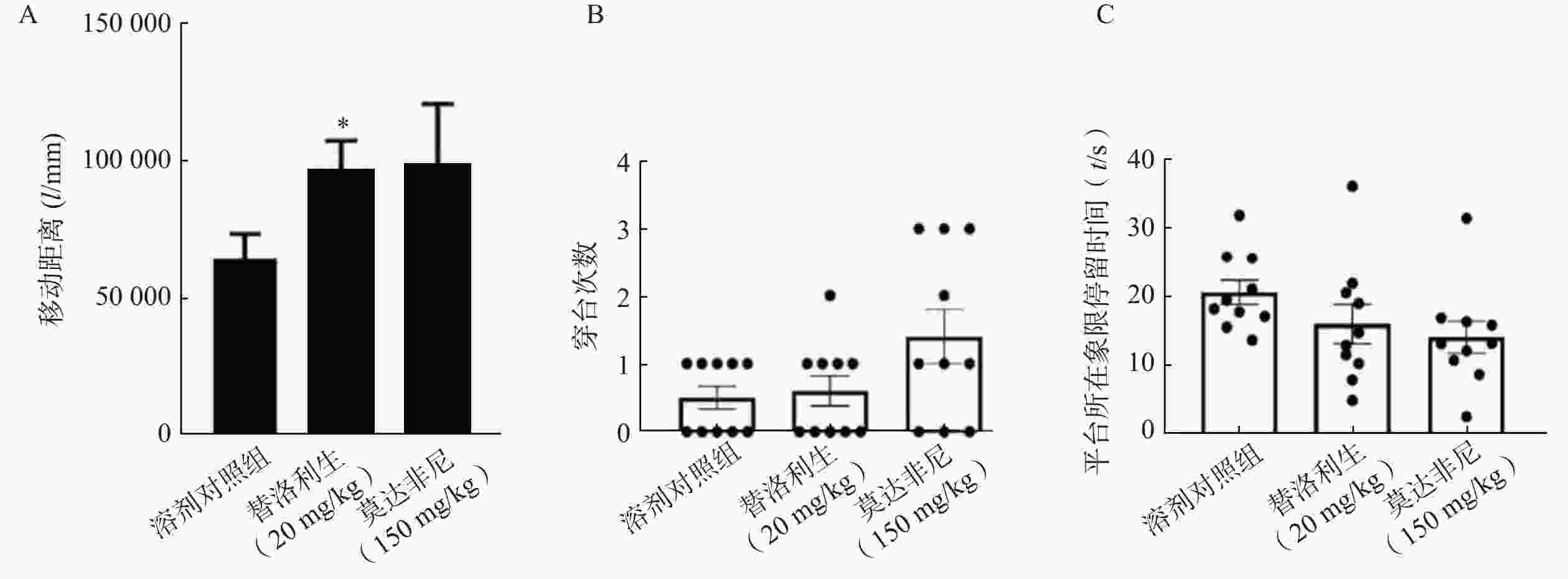
为探讨替洛利生对睡眠剥夺引起行为活性下降的干预效果,采用旷场实验检测小鼠在24 h (14:00–次日14:00)睡眠剥夺后的自发活动水平。结果显示,睡眠剥夺显著降低了小鼠的总移动距离(图2),而腹腔给予替洛利生组小鼠活动水平显著回升,移动距离比对照组增加了33%(n=10, P<0.05,图3A),呈现较为平稳的运动轨迹与探索行为。相比之下,莫达非尼组虽有上升趋势,但个体波动较大,且部分小鼠出现过度兴奋表现,差异未达统计学显著性(图3A)。
研究结果表明,替洛利生不仅具促醒能力,更能稳定提升清醒期的行为活性,有助于缓解睡眠剥夺所致的行为和运动抑制。
-
Morris水迷宫测试用于评估睡眠剥夺对空间记忆的影响及药物干预效果。隐藏平台训练结束后,小鼠经历24 h(14:00–次日14:00)睡眠剥夺,并腹腔给予替洛利生或莫达非尼,随后进行空间探索实验。
结果显示,替洛利生和莫达非尼组小鼠在穿越平台位置的次数上均较对照组略高,提示存在一定的记忆保持趋势,但3组间差异未达统计学显著水平(图3B,P>0.05)。目标象限停留时间亦未见显著差异(图3C),替洛利生组略高于莫达非尼组。
总体来看,替洛利生对睡眠剥夺引发的空间记忆损伤具有一定改善作用,但其认知改善效应仍需通过更多认知测试和剂量优化进一步验证。尤其睡眠剥夺导致的学习记忆损伤可能涉及更复杂的神经机制,强力促醒干预导致过度兴奋也不利于认知功能的恢复。
-
本研究评估了H3受体反向激动剂替洛利生在小鼠睡眠剥夺模型中的促醒和认知改善效应,并与莫达非尼做了比较,结果显示替洛利生在不同睡眠剥夺时程下均可显著延长清醒时间,并在一定程度上改善行为活动与空间记忆表现,具有潜在的促认知价值。
在6、12、24 h睡眠剥夺诱发的嗜睡条件下,相同剂量的替洛利生均能有效延长清醒时长,特别是在剥夺结束初期(前2~3 h )表现出明显和稳定的促醒优势。根据目前对该药的认识,其机制应与阻断H3自受体、增强组胺能系统活性有关,同时也可能通过H3异位受体间接促进乙酰胆碱、去甲肾上腺素等兴奋性神经递质释放,从而协同增强清醒状态的各项指标[7, 14, 18]。该作用模式在持续性及剂量稳定性方面具有一定优势,为后续临床转化提供了实验依据。
在行为学表现方面,替洛利生能有效恢复24 h睡眠剥夺模型小鼠的自发活动能力,且行为平稳性优于莫达非尼,提示其可能具有更优的安全性。同时,在空间记忆测试中,替洛利生组小鼠在穿台次数和目标象限停留时间上虽无显著差异,但表现出相较对照组更加有效的认知趋势。这提示在一定睡眠损伤条件下,促醒药物不仅可恢复行为活性,还可能对学习记忆产生积极影响。然而,认知改善效应未达统计学显著意义,可能与样本量、模型强度、剂量设定及对使用的行为测试敏感性等因素有关,需进一步优化实验设计和参数验证。
临床转化方面,替洛利生已获批用于治疗发作性睡病[12],其主要表现为白天过度嗜睡,机制上与本研究中的睡眠剥夺模型高度相关。本研究结果提示,该药物除促醒外或具备一定的认知改善潜力,未来可进一步拓展用于其他伴随嗜睡及注意力缺陷的疾病,如阻塞性睡眠呼吸暂停(OSA)或帕金森病等神经退行性疾病。此外,相较于莫达非尼,替洛利生诱发的过度兴奋行为较少、安全性更高[19],进一步提升其临床适用性。
未来研究可围绕以下方向开展:①优化剂量与给药途径:应进一步探索不同剂量、给药方式(口服或腹腔)对促醒及认知效应的差异,建立更稳定的量效关系。②拓展机制研究:结合分子生物学、脑电波谱与神经影像技术,深入揭示替洛利生在促醒与认知网络中的作用机制。③扩展行为学评估维度:未来研究可加入更多维度的认知任务(如注意力保持、执行功能与情绪反应)以全面评估其中枢作用。
综上所述,替洛利生在睡眠剥夺诱发的清醒缺失和嗜睡中展现出稳定的促醒效果,并可能具有一定的认知保护作用。结合其良好的安全性与现有临床应用基础,替洛利生有望成为新型认知障碍干预的重要候选药物。未来需在更大样本、多任务设计与临床研究中加以验证,推动其在睡眠与认知障碍领域的转化应用。
Alleviation of sleep deprivation-induced hyper sleepiness and cognitive impairment by pitolisant, a histamine H3 receptor inverse agonist
doi: 10.12206/j.issn.2097-2024.202506008
- Received Date: 2025-06-10
- Rev Recd Date: 2025-07-21
- Available Online: 2025-10-21
- Publish Date: 2025-10-25
-
Key words:
- pitolisant /
- H3 receptor inverse agonist /
- cognitive function /
- sleep deprivation /
- wakefulness promotion /
- pro-cognitive effects
Abstract:
| Citation: | ZHAO Yan, WEI Jun, MIAO Chaoyu. Alleviation of sleep deprivation-induced hyper sleepiness and cognitive impairment by pitolisant, a histamine H3 receptor inverse agonist[J]. Journal of Pharmaceutical Practice and Service, 2025, 43(10): 491-495. doi: 10.12206/j.issn.2097-2024.202506008 |







 DownLoad:
DownLoad: