-
疫苗的发展有力地降低了全球传染病的病死率和发病率。近年来,免疫学、分子遗传学和纳米技术的进步推动了疫苗研究的发展。为了进一步提高免疫原性,纳米疫苗在疫苗学领域的研究中应运而生。纳米疫苗是指大小在几纳米到几百纳米之间的颗粒状的疫苗。研究者根据需求,确定大小、形状等参数,把纳米疫苗制作成与免疫系统相似的目标,从而优化疫苗的生物分布以及与免疫细胞的相互作用,在疫苗领域展现出巨大的潜力[1–3]。在疫苗学应用中,纳米颗粒有3个主要功能,分别是作为佐剂、载体或者呈递平台。可以将这些功能中的一个或几个相互组合,以改善免疫反应。纳米颗粒作为颗粒佐剂[4]和核酸载体[5]已经取得了显著成功。疫苗开发中的纳米颗粒呈递平台是将抗原负载到颗粒中,通过增强抗原递呈细胞受体的运输和识别,促进免疫反应。然而,纳米疫苗的抗原递呈过程复杂、递送效率低的问题严重限制了其安全和有效性[6-7]。因此,基于纳米疫苗的体内递送过程特点,开发高效的靶向递送技术,是助其临床转化的重要环节。
纳米疫苗进入血液后将不可避免地吸附血浆蛋白,在表面形成蛋白冠。这些被吸收的蛋白质的构象和生物活性会主导纳米疫苗的体内性能[8-9]。所以,对生物纳米界面的精确调控为提高纳米疫苗的体内性能提供了新的可能。免疫球蛋白M(IgM)是个体发育中产生的第一个抗体,是免疫反应中分泌的第一个抗体,也是最有效的激活补体系统的免疫球蛋白之一[10]。免疫细胞可通过补体受体和IgM特异性受体(FcμR)与IgM结合。结合抗原的IgM可以与带有补体受体CD21/CD35的B细胞相互作用[11]。同时,FcμR主要表达在B细胞上,在宿主防御和体液免疫中具有重要作用[1,13]。由于IgM-补体-补体受体和IgM-FcμR途径都可以参与体液免疫应答,IgM可能是调节纳米疫苗与免疫细胞相互作用的关键血浆蛋白[1]。因此,需要主动操控IgM的结合来实现纳米疫苗的精准递送。
脂质体是一类技术成熟的纳米递送系统,也是一类具有巨大潜力的多功能药物载体[8]。经多年研究,脂质体取得了几项重大进展,如可实现靶向递送、可在体内“隐形化”以实现长循环、可在特定条件下响应等[14–16]。目前已成功上市的脂质体产品包括用于治疗癌症的Doxil,DaunoXome,Myocet 及抗真菌治疗的 Ambisome等[17–19]。
叶酸分子(FA)是B族水溶性维生素,参与机体核苷酸代谢。课题组前期研究发现叶酸可与IgM特异性结合[20-21]。叶酸作为结合IgM的靶分子修饰于纳米载体表面,可显著提高纳米疫苗的抗原递呈效率。
结合以上背景,本文构建了一种叶酸修饰的脂质体载体,负载模型抗原卵清蛋白制备成疫苗。通过静脉注射途径给药,考察叶酸脂质体疫苗通过在体内结合天然IgM,并在IgM的靶向赋能下,递呈至脾脏B细胞,刺激机体产生免疫应答能力。
-
R206D 旋转蒸发仪(上海申生);JY92-IIDN超声波细胞粉碎机(宁波新芝);Zetasizer nano 马尔文粒径仪(英国Malvern);H2050R-1台式高速冷冻离心机(湖南湘仪);FLAscent全波长酶标仪(美国Thermo Fisher Scientific);Tanon-
2500 凝胶成像系统(上海Tanon);MQD-A2R双层控温摇床(上海旻泉);超纯水系统(德国Sartorius);TCS SP5 Ⅱ激光共聚焦显微镜(德国leica); DYY-6C凝胶电泳仪(六一仪器)。 -
氢化磷脂酰胆碱(HSPC),mPEG2000-DSPE(上海艾伟拓);PBST干粉(上海泰坦);胆固醇(CHOL)(上海麦克林); FA-DSPE-mPEG
3400 (本实验室合成,HPLC检测纯度98.7%);快速银染试剂盒,TMB显色液,封闭液,BCA蛋白浓度测定试剂盒,辣根过氧化物酶(HRP)标记山羊抗小鼠IgG抗体,超敏ECL化学发光试剂盒(上海碧云天);HRP标记山羊抗小鼠IgM抗体(Abcam);FITC标记驴抗小鼠IgM抗体(Jackson Immuno);牛血清白蛋白(BSA),十二烷基硫酸钠(SDS)和Tris-base(国药集团);DiD荧光素,卵清蛋白(OVA,大连美伦);高结合力96孔酶联免疫吸附法(ELISA)板(Greiner)。 -
C57BL/6小鼠(雌性,6~8周龄,体重18~20 g,杭州子源实验动物科技有限公司,生产许可证号:SCXK(浙)2019-0004)、BALB/c小鼠 [雌性,6~8周龄,体重18~20 g,上海必凯科翼生物科技有限公司,生产许可证号:SCXK(沪)2023-0009],动物实验经过海军军医大学实验动物伦理委员会批准,并按照动物伦理学要求进行。
-
采用薄膜水化法制备叶酸脂质体疫苗。按照表1,精密称取处方量的HSPC,CHOL,DSPE-mPEG2000和FA-DSPE-mPEG
3400 ,用适量的氯仿溶解混合。减压旋蒸后,加入OVA的生理盐水溶液,60 ℃水化1 h,160 W探头超声10 min。超声后置于液氮与37 ℃水浴反复冻融数次。冻融后采用10 000 r/min低温高速离心30 min,弃上清液,并用生理盐水重悬两次。过0.22 μm微孔滤膜,得到叶酸修饰的脂质体疫苗,命名为FA-sLip/OVA,4 ℃保存备用。以相同的方法制备未经叶酸修饰的脂质体疫苗,命名为sLip/OVA。马尔文粒径仪测定其粒径、多分散性指数(PDI)及Zeta电位。脂质体疫苗类型 HSPC CHOL DSPE-
mPEG2000FA-DSPE-
mPEG3400 OVA sLip/OVA 9.58 3.19 3.19 0 5 FA-sLip/OVA 9.58 3.19 2.55 1.125 5 -
以BCA蛋白浓度测定试剂盒测定叶酸脂质体疫苗中OVA浓度。方法参考试剂盒说书,配制蛋白标准溶液和工作液,并加至96孔板中。取2 μl脂质体加至96孔板中,再用PBS稀释到20 μl,37 ℃孵育25 min,每个样品设置3个复孔。用酶标仪测定562 nm波长处的吸光度。以吸光度值(A)和标准溶液浓度绘制标准曲线,得到标准曲线方程,将样品吸光度值带入方程可得样品蛋白浓度,并通过以下公式计算OVA的包封率。
-
采用ELISA法检测叶酸脂质体疫苗与IgM的结合能力。50 μl脂质体铺于高结合力板中,4 ℃孵育过夜。弃去孔内液体,并用PBST洗涤。加入5 % BSA溶液于37 ℃孵育封闭1 h。加入梯度稀释的C57BL/6小鼠血清于 37 ℃孵育1 h。PBST洗涤后加入稀释的HRP标记的山羊抗小鼠IgM抗体, 37 ℃孵育1 h。PBST洗涤后,加入TMB显色液,室温避光反应,15 min后加入0.18 mol/L硫酸终止液。用酶标仪测450 nm处的吸光度值(A450)。
-
麻醉C57BL/6小鼠,眼眶取血,收集血清。分别取等体积的叶酸脂质体疫苗与血清至低蛋白吸附管中,37 ℃孵育1 h。加入预冷的PBS稀释至800 μl,14 000×g低温高速离心30 min,收集沉淀。将所得蛋白金属浴10 min,取出冷却至室温,进行SDS-PAGE电泳。电泳结束后,取出胶板。一方面按照银染试剂盒操作说明进行蛋白银染。另一方面,继续恒流400 mA转膜25 min。电泳完毕取出PVDF膜,将膜置于封闭液中,再置于摇床上室温缓慢封闭1 h。倒尽封闭液,加入稀释的HRP标记的山羊抗小鼠IgM抗体,置于摇床上37 ℃孵育1 h。抗体孵育完后,用PBST快速洗涤。洗涤完成后加入ECL发光显色液,避光反应后用成像仪曝光显色。
-
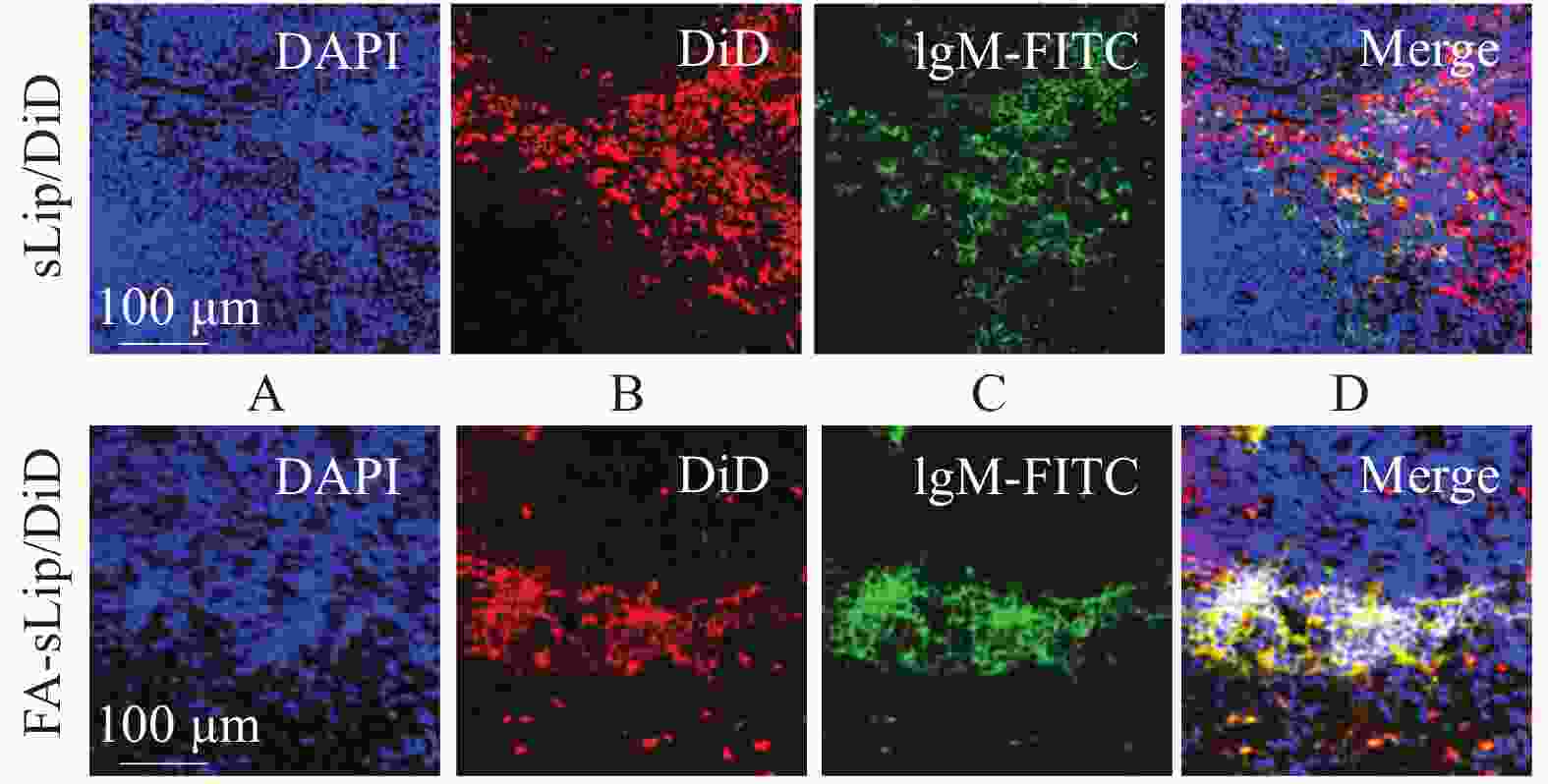
按表1的处方并加入适量的DiD荧光素,氯仿溶解并成膜。并照“2.1”项下方法制备负载DiD的脂质体,命名为sLip/DiD和FA-sLip/DiD。经尾静脉注射至BALB/c小鼠体内,并于给药4 h后收集脾脏。脾脏冷冻切片,DAPI染核,FITC标记的IgM抗体标记小鼠脾脏边缘B细胞,利用激光共聚焦观察脂质体在脾脏中的分布。
-
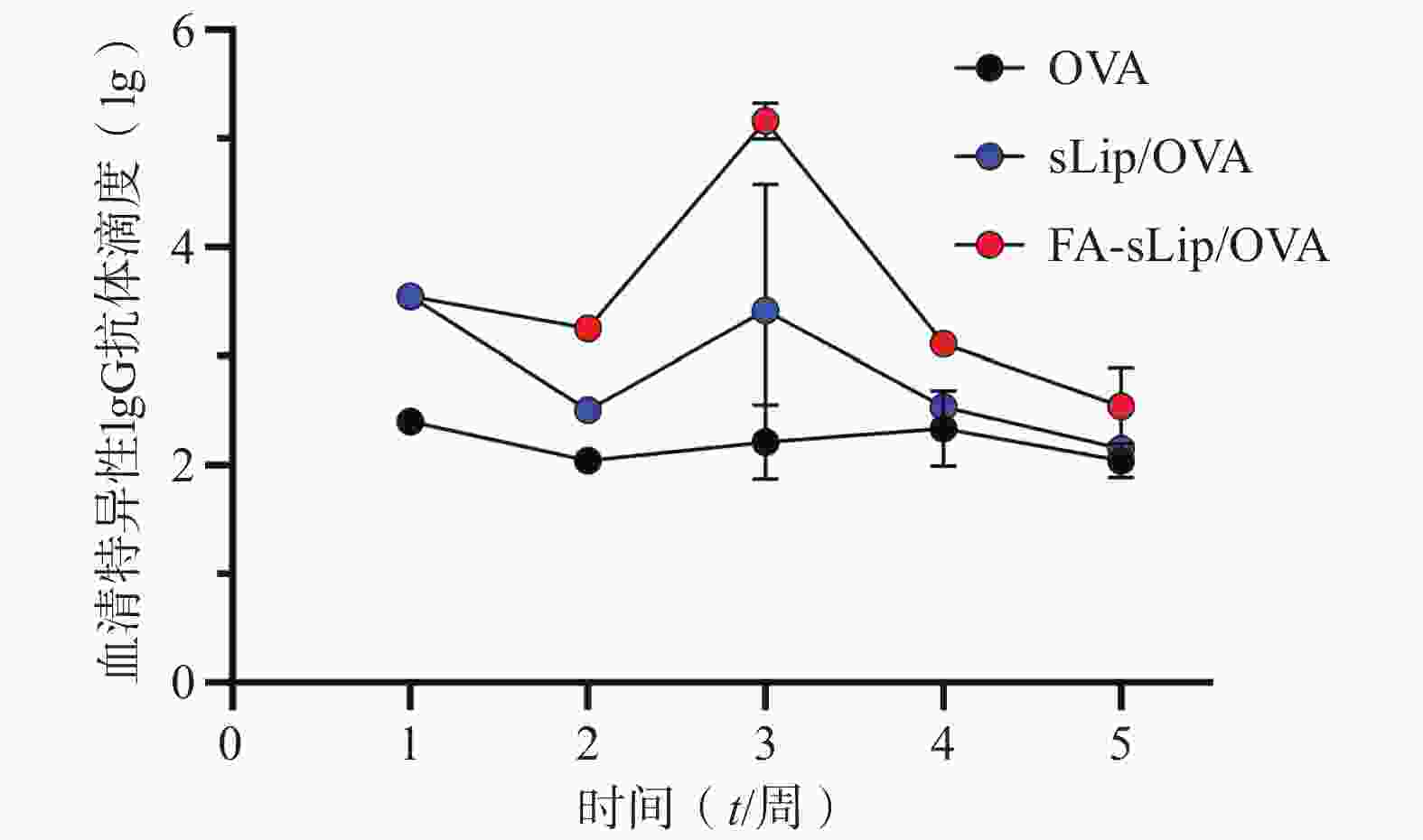
C57BL/6小鼠随机分为3组,每组5只,分别为Free OVA、sLip/OVA和FA-sLip/OVA。通过静脉免疫,按20 μg OVA/只的剂量给药,7 d给药1次,共免疫3次。小鼠末次免疫接种完成后第7天取血,3 500 r/min低温离心15 min,获得血清。ELISA法测定小鼠血清中的特异性IgG抗体滴度。
-
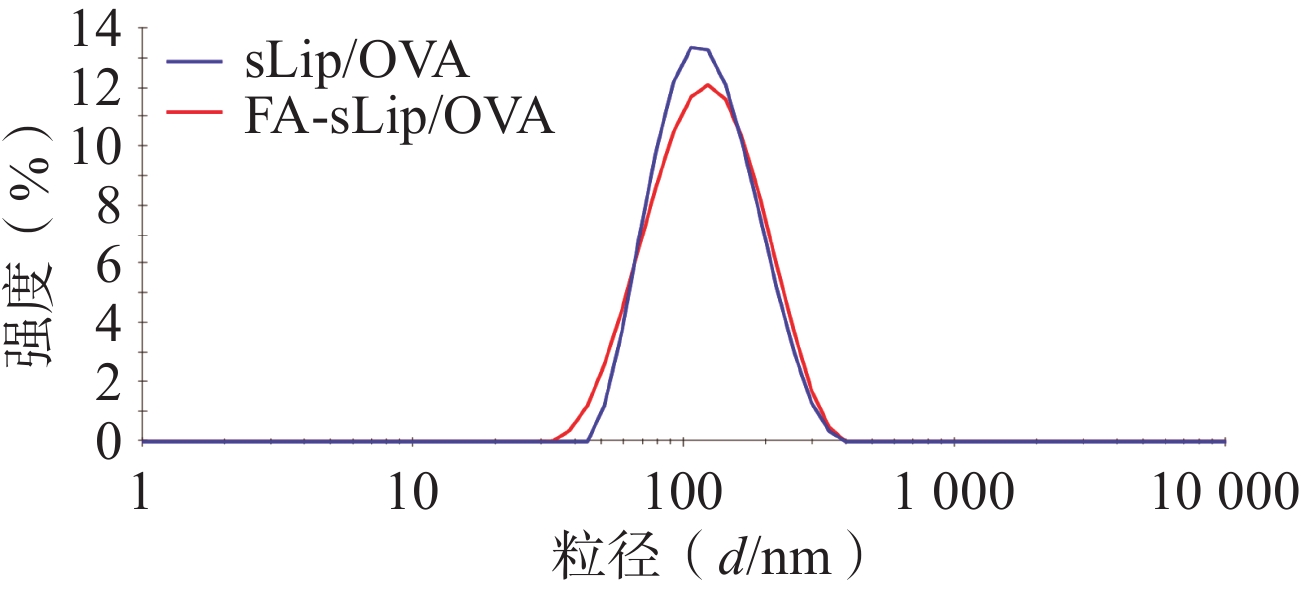
制得未经叶酸修饰的脂质体疫苗(sLip/OVA),溶液呈白色乳光,平均粒径约103.7 nm,分布良好。叶酸修饰的脂质体疫苗(FA-sLip/OVA),溶液呈现淡黄色乳光,粒径稍有增大,约117.2 nm(表2)。叶酸脂质体疫苗的粒径分布见图1。
粒径(d/nm) PDI Zeta 电位(ζ/mV) sLip/OVA 103.7±1.0 0.143±0.014 −7.387±0.323 FA- sLip/OVA 117.2±2.1 0.163±0.021 −8.023±0.917 -
通过BCA试剂盒测得的标准曲线方程Y=0.221 2X+0.110 8,线性关系良好(r=0.997 4)。经计算可得,FA-sLip/OVA的OVA含量为1.35 mg/ml,包封率为26.94%。sLip/OVA的OVA含量为1.39 mg/ml,包封率为27.76%。
-
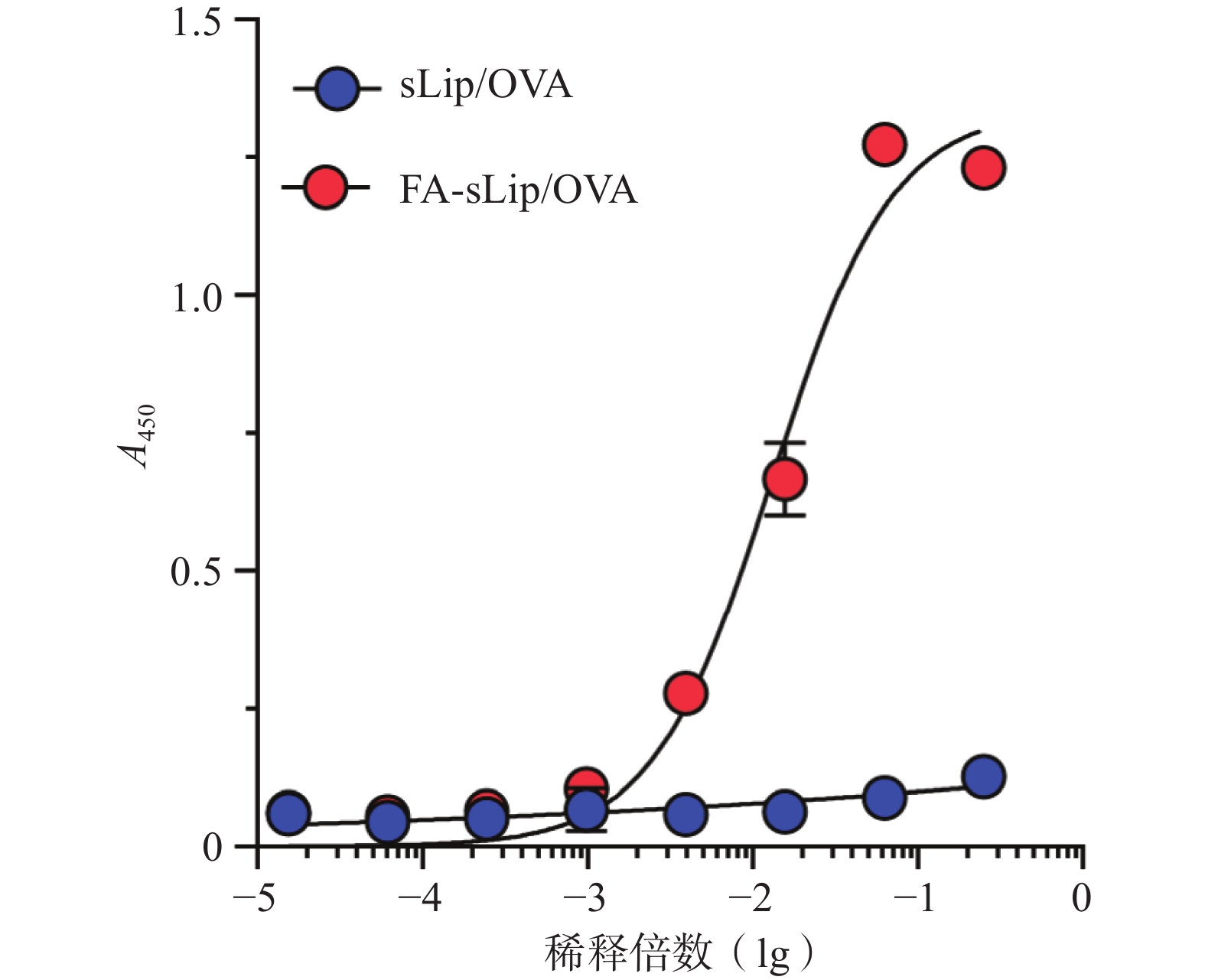
脂质体疫苗与血清中IgM相互作用结果如图2所示,脂质体通过叶酸修饰,显著增强了与IgM的结合力。
-
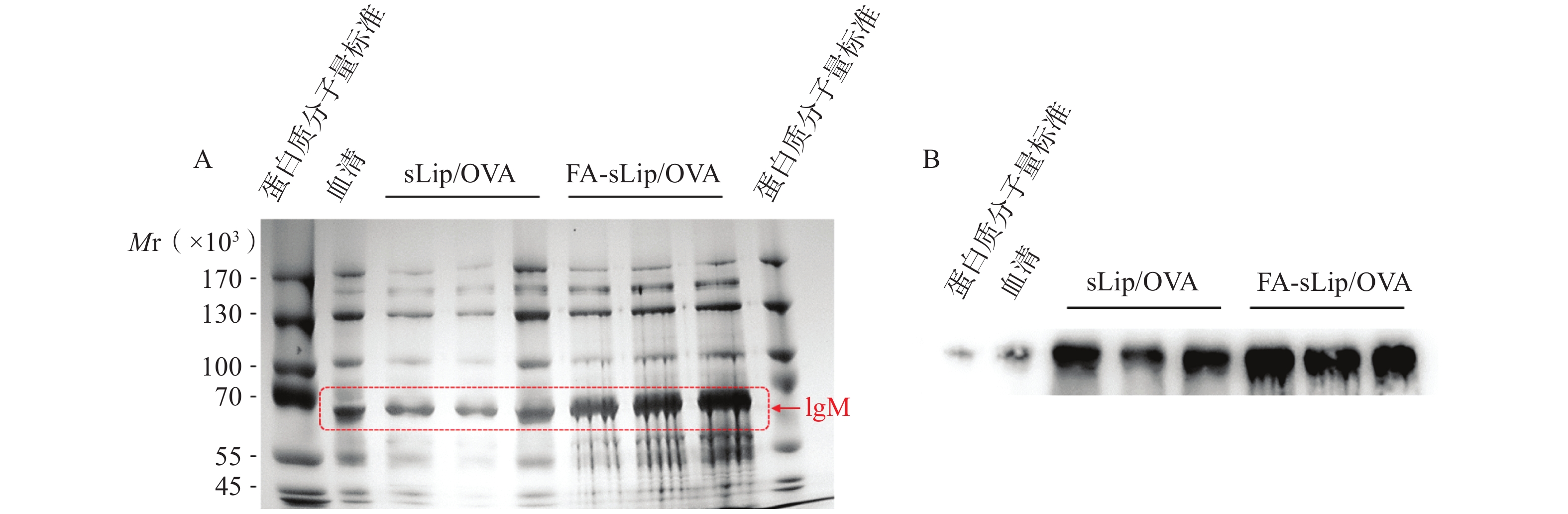
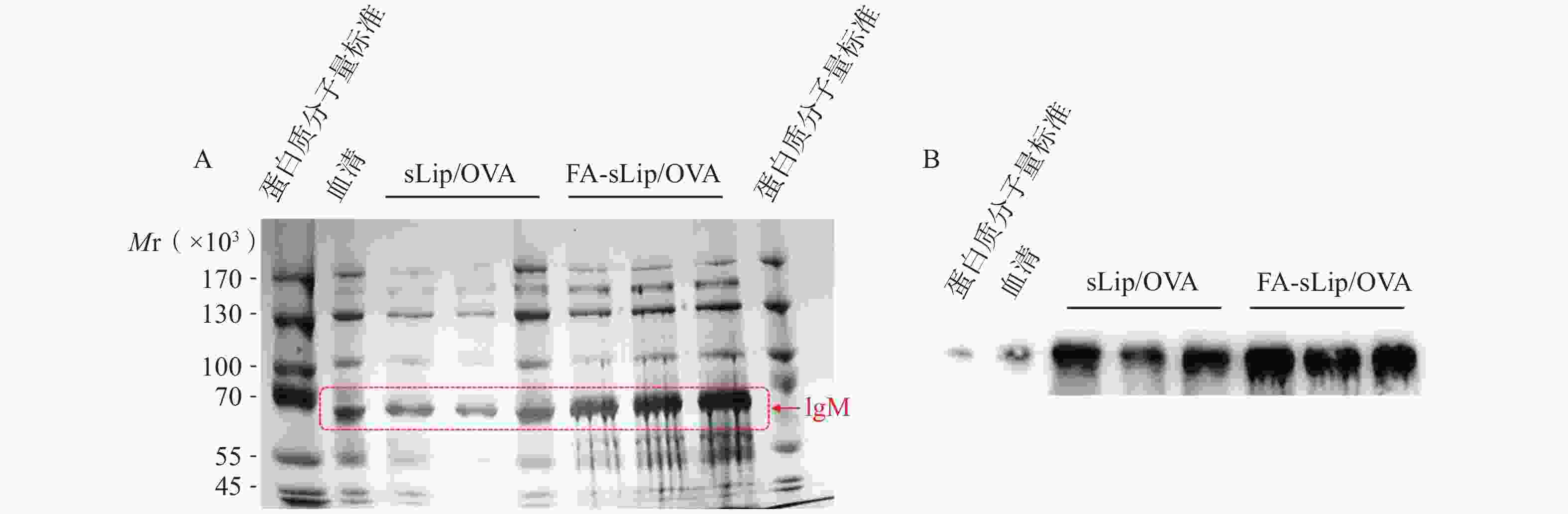
SDS-PAGE分析脂质体疫苗与血清孵育得到的蛋白冠,在分子量约为72 000处可见明显的蛋白条带。且FA-sLip/OVA组的IgM吸附量多于sLip/OVA的IgM吸附量(图3A)。Western Blot同样证实FA-sLip/OVA组的IgM吸附量多于sLip/OVA组的IgM吸附量(图3B)。
-
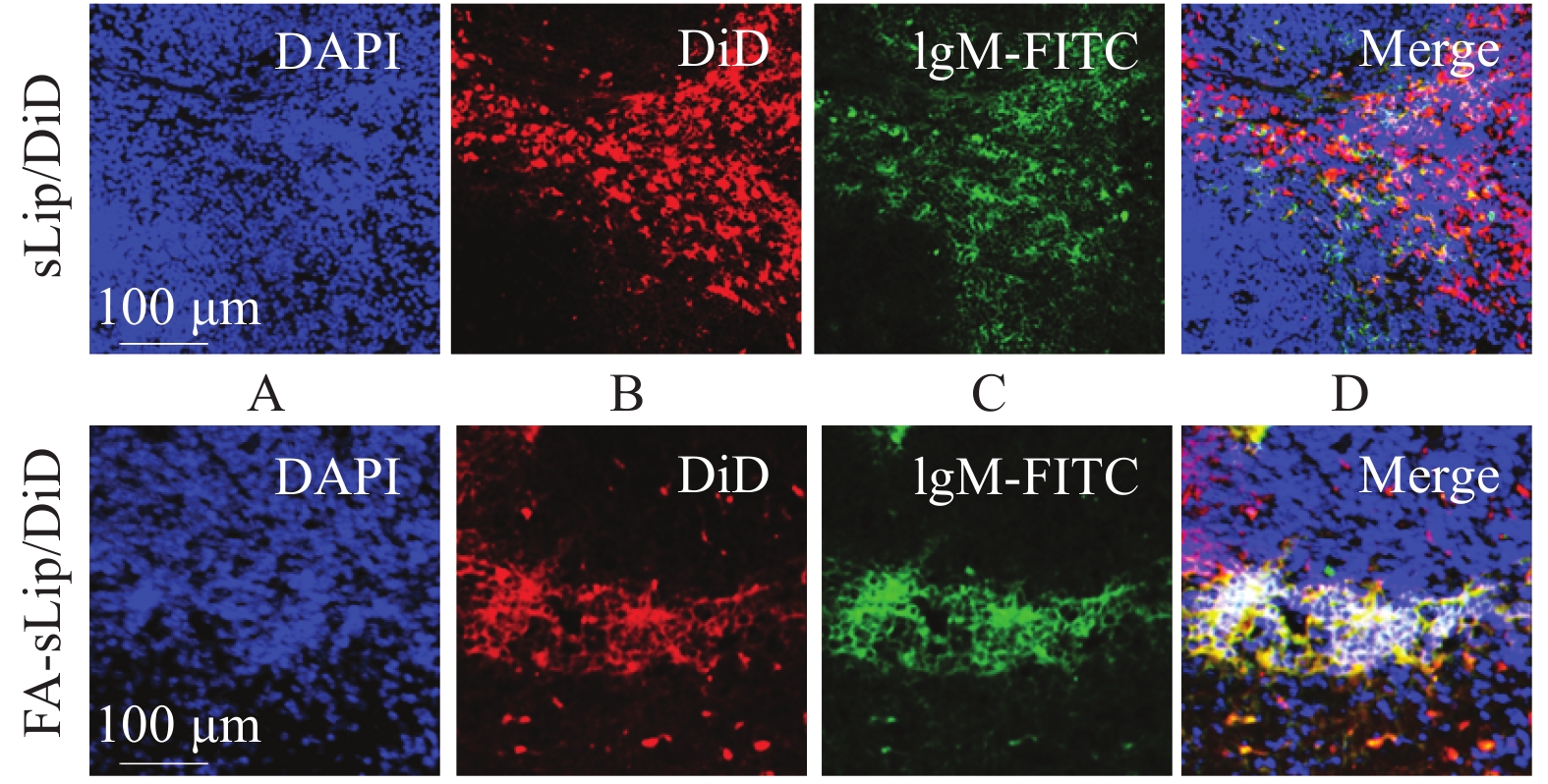
图4显示了脂质体疫苗在体内对脾脏B细胞的初步靶向性。通过激光共聚焦观察小鼠脾脏冷冻切片,观察叶酸脂质体的脾脏内分布,结果显示,叶酸脂质体更多地分布在脾脏的边缘区域(图4B),与IgM抗体标记的脾脏边缘B细胞(图4C)具有较好的重叠(图4D)。以上结果提示,叶酸脂质体具有良好的脾脏边缘B细胞靶向性。
-
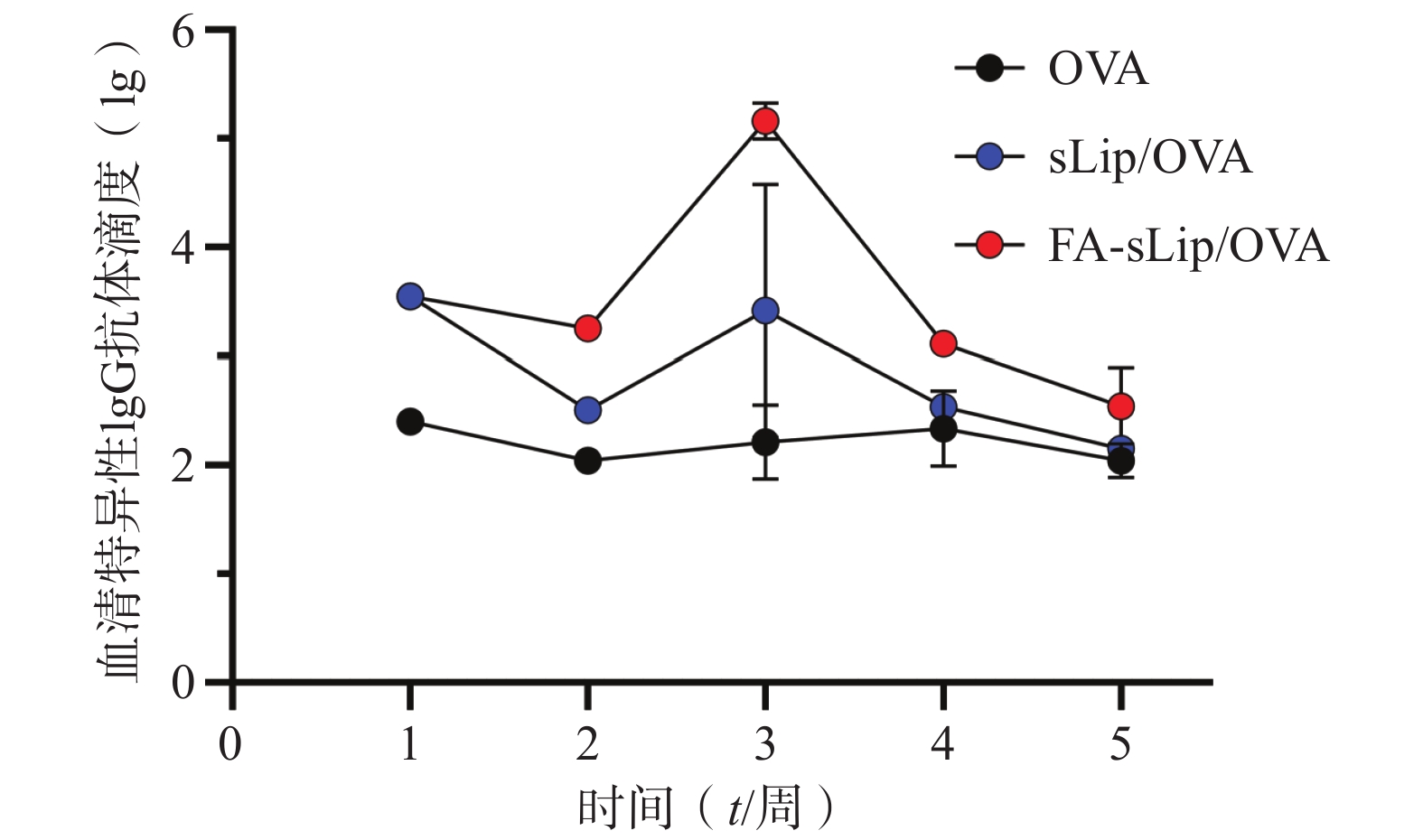
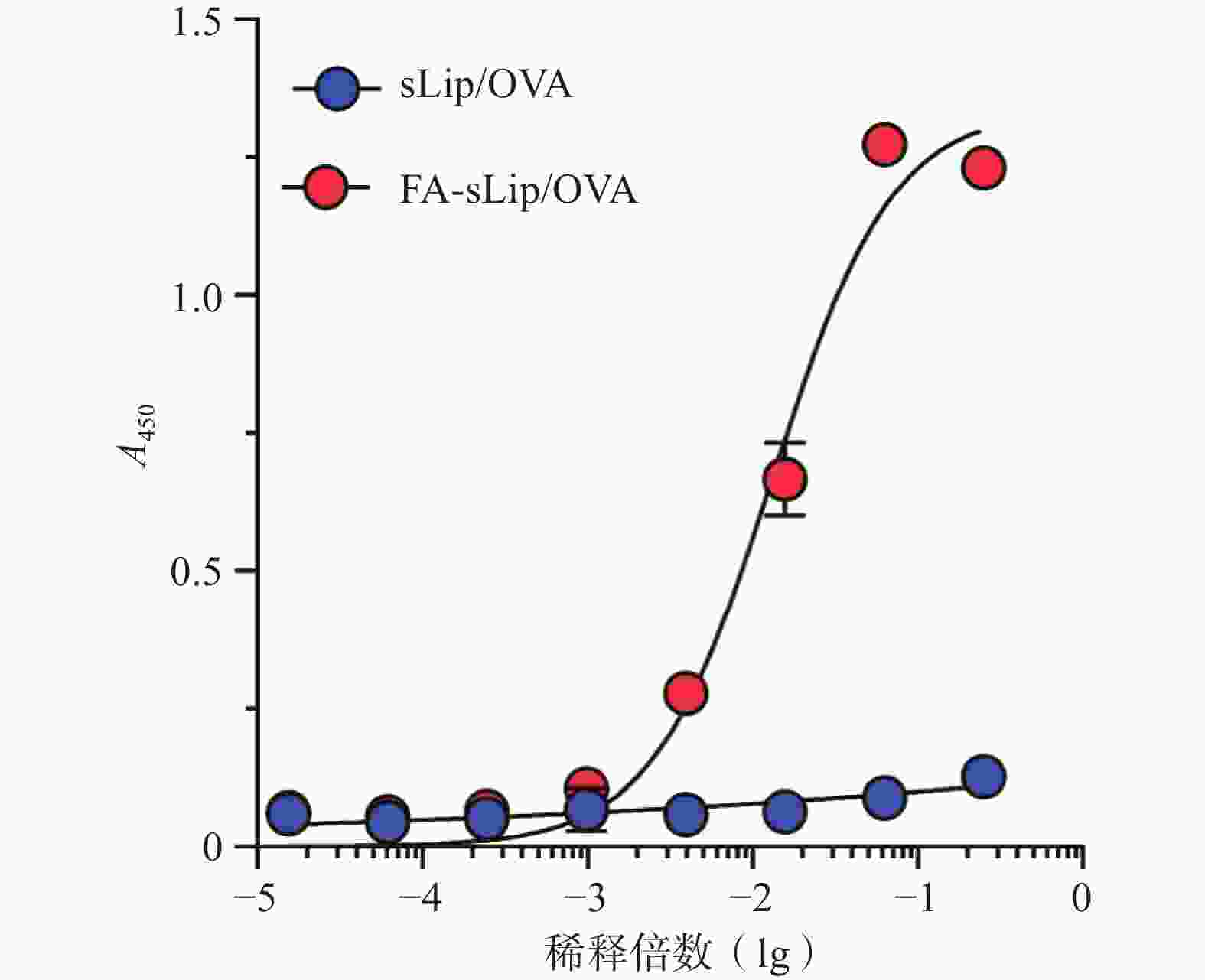
通过间接酶联免疫吸附测定法检测小鼠血中特异性IgG抗体,结果显示如图5。结果表明脂质体疫苗给药后,能在一定时间内保持较高的抗体滴度。其中,叶酸修饰的脂质体疫苗可以刺激机体产生更高的抗体滴度水平。
Preparation and evaluation of folic acid-modified liposomal vaccines based on natural IgM adjuvant
doi: 10.12206/j.issn.2097-2024.202401060
- Received Date: 2024-01-25
- Rev Recd Date: 2024-05-24
- Available Online: 2025-11-19
- Publish Date: 2025-11-25
-
Key words:
- vaccines /
- liposomes /
- nano-vaccine /
- IgM functionalization /
- folic acid
Abstract:
| Citation: | LIU Anze, HE Yingying, WANG Huan, LU Ying. Preparation and evaluation of folic acid-modified liposomal vaccines based on natural IgM adjuvant[J]. Journal of Pharmaceutical Practice and Service, 2025, 43(11): 555-559. doi: 10.12206/j.issn.2097-2024.202401060 |







 DownLoad:
DownLoad: