-
脓毒症(sepsis)是由微生物感染引发的宿主免疫反应失调,可导致休克、多器官功能障碍进而危及生命[1]。流行病学调查显示,脓毒症的死亡率约40%,全球每年约530万人死于脓毒症,占所有医院内死亡人数的近1/2,其发病率和死亡率之高,严重威胁人类健康和生命[2-3]。目前临床对脓毒症的治疗仍然以非特异性治疗为主,早期应用广谱抗生素,晚期采用器官功能支持疗法,但因缺乏特异性且疗效不佳,因此探索新的治疗手段迫在眉睫。
干细胞(stem cells)是一类具有自我更新和多向分化潜能的细胞,在特定条件下可分化为多种特定类型的细胞,如血液细胞、神经细胞、成骨细胞等,在再生医学领域展现出巨大潜力。干细胞治疗作为新型的治疗手段,在心脑血管疾病[4-5]、肝脏疾病[6]及肺部疾病[7]等领域展现出显著的治疗潜力,已进入临床试验阶段。目前大部分研究将干细胞的治疗作用归因于间充质干细胞(mesenchymal stem cells,MSCs),但造血干细胞(stem cells,HSCs)及干细胞衍生物细胞外囊泡(extracellular vesicle,EV)也表现出免疫调节等重要功能,具有临床应用前景。有研究表明,干细胞能影响脓毒症的病理进程,减轻脓毒症的严重程度[8]。本文综述干细胞的生物学特性及其治疗脓毒症的最新研究进展,以期为脓毒症的干细胞治疗提供参考。
-
自我更新是干细胞通过分裂维持自身群体稳定性的过程,是其在再生医学应用中不可或缺的关键特性,该过程依赖于干细胞对细胞周期的精确调控及其多能性的维持。自我更新过程涉及Wnt、Notch和PI3K/Akt等多种信号通路,这些通路通过调节细胞周期和分化相关基因的表达来影响干细胞的命运[9]。此外,表观遗传修饰在调控干细胞自我更新和定向分化中也发挥重要作用[10]。
-
干细胞具有多向分化潜能,可在特定条件下分化成多种类型的细胞,执行不同的功能。根据分化能力可划分为全能干细胞(totipotent stem cells,TSCs)、多能干细胞(pluripotent stem cells,PSCs)、成体干细胞(adult stem cells,ASCs)和单能干细胞(unipotent stem cells,USCs)。TSCs可分化成个体的所有细胞类型,形成一个完整的个体;PSCs可以分化成构成个体的所有细胞类型,但无法发育为完整个体,在合适的条件下,PSCs可分化成外胚层(表皮细胞、视网膜细胞)、中胚层(血管内皮细胞、成骨细胞)和内胚层(肝细胞、肺泡细胞和血细胞)的细胞;ASCs可以分化成多种细胞类型,但不能分化成构成个体的所有细胞类型(如HSCs分化成造血系统的所有细胞类型);USCs只能分化成终末细胞(如骨骼肌干细胞只能分化成横纹肌细胞)。这一特性为组织修复、疾病治疗及器官再生奠定了重要基础[11]。
-
干细胞归巢是指循环系统中的干细胞定向迁移至靶组织并定植的过程。这一过程类似于炎症部位白细胞的募集反应,体现了干细胞及祖细胞向受损组织迁移并参与修复的能力[12]。
当组织遭遇损伤时,机体通过分泌多种生长因子、趋化因子和黏附分子,如多效素(pleiotrophin)、血管内皮生长因子(vascular endothelial growth factor,VEGF)、基质细胞衍生因子-1(stromal cell-derived factor-1,SDF-1)、血管细胞黏附分子-1(vascular cellular adhesion molecule-1,VCAM-1)、整合素(integrin)等,诱导干细胞向损伤的组织部位定向迁移并定植[13]。由此可见,干细胞归巢能力在维持造血稳态、提升移植疗效以及调控病理状态下细胞迁移方面均具有重要意义。
-
低免疫原性是指细胞或分子激活宿主适应性免疫反应(包括体液免疫与细胞免疫)的能力较弱。该特性意味着干细胞移植后不易被宿主免疫系统识别并清除,显著降低免疫排斥反应风险。
研究表明,干细胞表面缺乏主要组织相容性复合体Ⅱ类分子(major histocompatibility complex-Ⅱ,MHC-Ⅱ)及共刺激分子的表达,导致其难以被宿主免疫系统识别并清除[14]。此外,干细胞还可以通过分泌多种免疫调节因子,如转化生长因子-β(transforming growth factor β,TGF-β)和前列腺素E2(prostaglandin E2,PGE2),进一步抑制免疫细胞活化,从而有效降低适应性免疫反应[15]。
-
免疫调节是机体维持免疫稳态的核心机制,其本质在于协调免疫应答与免疫耐受状态之间的动态平衡。该过程通过机体精确调控,确保免疫系统既能高效清除病原体及异常细胞,又可避免因过度激活导致自身组织损伤或慢性炎症[16]。
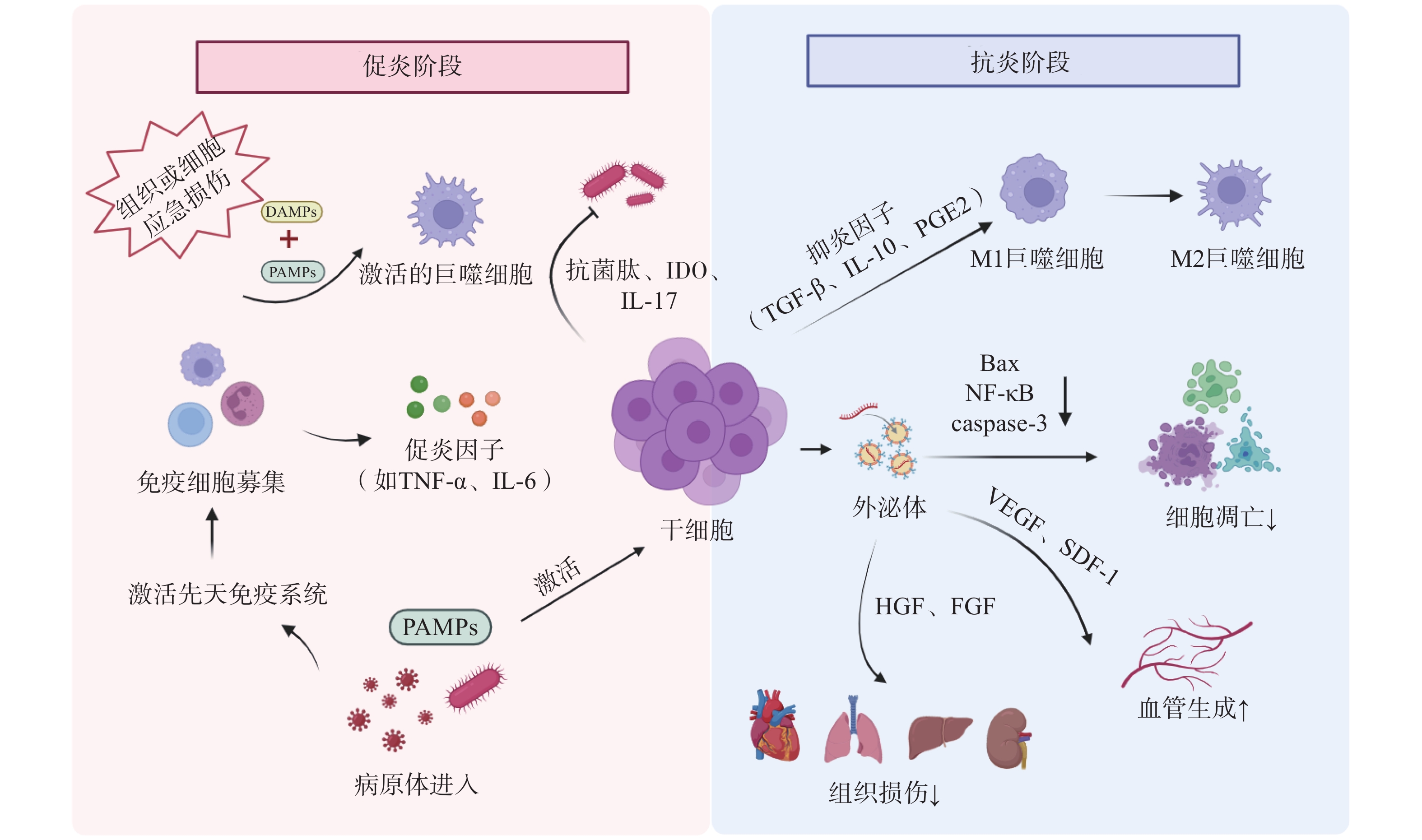
干细胞通过下调促炎因子如肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)、白细胞介素-1β(interleukin-1β,IL-1β)和上调抗炎因子如IL-10、PGE2、TGF-β等的表达,减轻机体过度炎症反应和免疫细胞的过度活化[14]。此外,干细胞通过外泌体(exosomes,Exo)及细胞间直接接触等方式,调节T细胞、B细胞、树突状细胞、巨噬细胞、自然杀伤细胞等免疫细胞的增殖、分化、凋亡及抗原递呈能力,最终协同维持机体免疫稳态(见图1)[15]。
-
旁分泌效应(paracrine effect)指干细胞通过分泌生物活性分子(如细胞因子、生长因子、Exo等),以非接触方式调控邻近细胞功能,从而促进组织修复或介导免疫调节的重要生物学过程。相关研究表明,人胎盘干细胞可分泌多种生物活性分子,如VEGF、成纤维细胞生长因子(fibroblast growth factor,FGF)、肝细胞生长因子(hepatocyte growth factor,HGF)等,通过激活内皮细胞迁移与增殖,加速烧伤创面的血管新生,最终促进伤口愈合[17-18]。
-
干细胞表面表达多种模式识别受体(pattern recognition receptors,PRRs),包括Toll样受体(Toll-like receptors,TLR)、核苷酸结合寡聚结构域样受体2(nucleotide binding oligomeric domain like receptor 2,NOD2)等,此类受体可特异性识别病原体相关分子模式(pathogen associated molecular patterns,PAMPs),通过触发炎性细胞因子和抗菌肽等效应分子的释放,直接或间接介导抗微生物免疫应答[18]。
-
炎症稳态失衡贯穿脓毒症发生发展的全过程。机体的免疫活化有利于清除入侵病原体,但持续的炎症反应则会导致组织损伤,过度的炎症反应更可能诱发机体的免疫功能紊乱。
宿主对侵袭性病原体的急性应答可诱导巨噬细胞吞噬病原体,并触发一系列促炎细胞因子如TNF-α、IL-6、IL-1β、干扰素γ(interferon-γ,IFN-γ)、高迁移率族蛋白B1(high mobility group protein B1,HMGB1)等的大量分泌,进而引起细胞因子风暴及先天免疫系统的过度激活。Shen等[19]研究发现,脂肪干细胞(adipose derived stem cells,ADSCs)外泌体可通过降低盲肠结扎穿刺(cecal ligation and puncture,CLP)脓毒症模型小鼠血清中TNF-α和IL-1β的水平,显著减轻全身炎症反应;同时通过清除活性氧(reactive oxygen species,ROS)抑制氧化应激损伤,从而缓解脓毒症相关急性肺损伤。Sato等[20]研究表明,人羊水干细胞具有MSCs特性,在预防性治疗中,通过与腹腔巨噬细胞聚集体相互作用,下调脂多糖(lipopolysaccharide,LPS)诱导的新生大鼠内毒素血症模型血清TNF-α、IFN-γ等促炎因子水平,显著提高存活率。此外,Khosrojerdi等[21]研究表明,MSC衍生外泌体(MSC-derived exosomes,MSC-Exo)联合亚胺培南可显著提升CLP小鼠脾及肠系膜淋巴结中调节性T(Treg)细胞的比例和数量,通过扩增Treg细胞抑制过度炎症,重建免疫稳态,最终改善脓毒症免疫缺陷状态。
-
在脓毒症的病理过程中,细胞凋亡是免疫抑制的驱动因素。该过程涉及多种关键免疫细胞的功能耗竭与异常死亡,如T细胞耗竭、淋巴细胞亚群凋亡及树突状细胞数量锐减,共同导致免疫耐受。干细胞疗法可通过调节B淋巴细胞瘤-2(B-cell lymphoma-2,Bcl-2)蛋白家族平衡、线粒体功能、内质网应激及炎症信号通路多维度调控细胞凋亡途径,成为干预脓毒症的新策略[22]。
Bcl-2作为经典抗凋亡蛋白,与促凋亡成员Bax(Bcl-2 associated X protein,Bax)共同构成Bcl-2蛋白家族的核心调控模块。该家族通过调控线粒体外膜通透化,介导细胞色素C释放并激活下游半胱天冬酶-3(caspase-3),从而启动程序性死亡通路。其中半胱天冬酶-3的高效活化是细胞不可逆凋亡的关键执行节点。Akhondzadeh等[23]研究证实,在CLP诱导的脓毒症大鼠模型中,海马体神经元损伤与记忆检索功能障碍显著相关,而脂肪来源的MSCs干预可显著降低右侧海马体组织中半胱天冬酶-3表达水平,并下调Bax/Bcl-2比值,有效缓解脓毒症相关的海马体病理损伤,提示MSCs具有一定神经保护潜力。
-
内皮细胞(endothelial cells,ECs)作为血管及淋巴管内皮层的广泛分布结构,对维持血管稳态、调控炎症反应和保障屏障完整性至关重要。脓毒症可诱导内皮功能障碍和血管反应失调,驱动全身性微循环衰竭,最终进展为多器官功能障碍综合征(multiple organ dysfunction syndrome,MODS)。
Laddha等[24]研究表明,MSCs能够通过外泌体的旁分泌作用递送VEGF、FGF、HGF等生长因子,直接激活内皮细胞增殖和迁移,提升缺血组织的毛细血管密度。Cóndor等[25]研究发现,人沃顿果冻来源的MSCs(wharton jelly-derived MSCs,WJ-MSCs)可显著提高CLP脓毒症模型大鼠VEGF表达水平,提示WJ-MSCs可能通过改善CLP大鼠的内皮功能障碍缓解脓毒症病理进程。
-
脓毒症的核心病理过程表现为炎症与免疫的动态失衡,而病原微生物的持续存在是驱动这一恶性循环的关键因素。入侵病原体能够释放PAMPs,如LPS、脂磷壁酸等被宿主PRRs识别,通过激活核因子κB(nuclear factor-κB,NF-κB)等关键信号通路触发炎症级联反应,导致全身性炎症反应[22]。
干细胞治疗可显著降低脓毒症小鼠血液及组织器官中的病原体负荷。Jerkic等[26]研究表明,啮齿动物PSCs定向分化产生的胚胎源性“腹膜样”巨噬细胞能有效清除脓毒症小鼠肝、脾和肺部的细菌,进而降低脓毒症的严重程度,提高脓毒症小鼠存活率。此外,MSCs可通过分泌抗菌肽、吲哚胺2,3-双加氧酶(indoleamine 2,3-dioxygenase,IDO)、IL-17等物质直接或间接杀灭病原体[27],同时增强免疫细胞对病原体的杀伤能力,进而降低宿主荷菌量,减轻脓毒症症状,最终缓解脓毒症病理进展[28]。
-
脓毒症病理机制之一是宿主对病原体感染的免疫反应失调,最终引发MODS。PAMPs通过PRRs激活免疫细胞,触发由NF-κB等信号通路介导的炎症级联反应,促使TNF-α、IL-1β等促炎因子过量释放,形成“炎症因子风暴”。血液中大量的炎性细胞因子促使炎症扩散以及免疫细胞过度激活,介导组织损伤;同时通过诱导线粒体功能障碍加剧多器官功能衰竭[22]。
心肌抑制是指在严重感染、创伤等病理状态下,心脏收缩和舒张的功能障碍,是脓毒症最常见的并发症之一,其本质是炎症介导的心肌细胞损伤。Wu等[29]研究表明,在LPS诱导的内毒素血症小鼠模型中,MSCs可显著降低血清及心肌组织中IL-1β、IL-6、TNF-α等促炎因子水平,有效改善心功能指标。值得注意的是,LPS刺激后小鼠射血分数(ejection fraction,EF)显著降低,而MSCs治疗可逆转此病理改变并恢复EF至生理水平。肝功能障碍同样高发于脓毒症患者(39.9%),其中8.5%进展为急性肝衰竭。Miao等[30]研究表明,骨髓MSCs(bone marrow MSCs,BM-MSCs)可通过分泌PGE2激活肝巨噬细胞,促使后者释放抗炎因子IL-10,进而抑制NOD样受体热蛋白结构域相关蛋白3(NOD-like receptor family protein 3,NLRP3)炎性小体组装与活化,改善LPS刺激后脓毒症小鼠的肝功能。
-
MSCs最初从骨髓分离,现也可从脂肪、脐带、胎盘等组织获取。不同来源MSCs各有特点:脂肪组织来源的MSCs获取相对便捷,细胞产量较高[31];而脐带、胎盘等围产期组织来源的MSCs通常增殖能力更强。MSCs可通过静脉、腹腔、肺部途径有效递送,给药途径对治疗效果起重要作用。MSCs在脓毒症动物模型中具有调节免疫、促进病原体清除、恢复器官功能和降低死亡率等治疗潜力。MSCs治疗作为一种潜在的脓毒症新疗法,可通过同种异体疗法发挥其相对免疫优势,从而达到缓解和治疗脓毒症的目的。
Alp等[32]开展的临床研究纳入了10例脓毒症患者,证实静脉输注脂肪来源的MSCs(剂量为1×106 细胞/kg)可显著提高早期脓毒症患者的生存率。He等[33]报告的Ⅰ期剂量递增试验(n=15,随访18个月)显示,脐带来源的人MSCs(human MSCs,hMSCs)静脉输注后,脓毒症患者第8天炎症标志物(IL-6、IL-8、TNF-α)水平下降,但未观察到明显的剂量效应(ChiCTR-TRC-14005094)。另一项纳入32名健康受试者的Ⅰ期临床试验表明,静脉输注高剂量(4×109 细胞/kg)同种异体脂肪MSCs后,人类内毒素血症模型中抗炎因子IL-10、TGF-β的释放增加(NCT02328612)。此外,一项Ⅱ期随机对照研究纳入66例严重感染性休克患者,以评估异源MSCs对脓毒症患者死亡率及器官衰竭的影响(NCT02883803),该研究有望为MSCs用于脓毒症患者的治疗提供试验依据。
-
HSCs移植( HSCs transplant,HSCT)是最常见的器官移植类型。Hamilton等[34]的回顾性研究分析提示,接受HSCT的患者相较于未接受移植的患者发生菌血症时死亡率较低。然而,接受HSCT患者本身处于免疫抑制状态,是脓毒症的高危人群,且一旦发生脓毒症往往预后不良[35],因此该发现更加凸显了HSCT在脓毒症中的治疗潜力。
-
EV根据其直径分为Exo、微泡和凋亡小体,可来自干细胞。MSC-Exo是一种无细胞疗法,它直接利用细胞分泌的生物活性成分来发挥治疗作用,既能规避细胞移植的风险,又具有良好的组织穿透性。相较于传统细胞疗法,MSC-Exo不需要冷冻保护剂,具有低免疫原性、低毒性、在血液中相对稳定等优点[36]。现有证据表明,MSCs的部分治疗效应可能由MSC-Exo介导[37-39]。一项正在进行的临床试验(NCT04850469)纳入了某院2年内所有因严重感染入院的儿童,评估MSC-Exo在重症感染儿童中的应用和治疗效果,该研究有望为MSC-Exo用于重症感染儿童的治疗提供试验依据。
-
脓毒症是由感染引发的全身性反应失调,进而导致多器官功能障碍,临床上属于急危重症。其病理核心包括炎症风暴(促炎因子如TNF-α、IL-6等的过度释放)、免疫紊乱(抗炎与促炎反应失衡)、微循环障碍及多器官损伤等多个环节。发病过程始于病原体过度激活免疫细胞,进而触发失控的炎症级联反应,最终导致组织缺氧、细胞凋亡和器官功能障碍[22]。
干细胞疗法作为再生医学的重要分支,近年来在多种疾病的治疗中展现出巨大潜力,其在脓毒症治疗中的应用同样具有广阔的前景。干细胞凭借其抗菌与抗炎活性,能够直接清除病原体并调节由微生物感染引发的早期炎症反应。同时,干细胞作为有效的免疫调节剂,可以通过抑制过度的炎症或防止免疫抑制,帮助机体恢复免疫稳态。此外,干细胞具有归巢与分布能力,可实现对炎症和损伤部位的靶向治疗,有助于发挥全身性治疗效应。在脓毒症后期,宿主常出现免疫抑制,进而导致MODS,此时干细胞的器官保护作用显得尤为重要。综上所述,干细胞在脓毒症的辅助治疗中展现出明确的转化价值与临床潜力[8]。
目前,干细胞的临床研究已取得初步进展。多项Ⅰ/Ⅱ期临床试验表明,静脉输注MSCs可以降低脓毒症患者体内IL-6、IL-8、TNF-α的水平,促进IL-10、TGF-β的释放,并提高早期脓毒症患者的生存率[32-33, 40]。然而,干细胞的临床应用仍面临诸多挑战。干细胞在脓毒症微环境中的分化行为、旁分泌因子互作等机制尚未明确;尽管目前未见干细胞治疗相关的严重不良反应,但潜在风险(如血栓形成)仍然需要警惕。此外,干细胞制备成本较高、保存技术复杂,最佳给药剂量与浓度、注射时间可能在不同研究中存在差异,冷冻的MSCs相较于新鲜MSCs免疫调节的功能可能减弱等问题仍有待进一步研究解决[41]。未来研究尚需深入探讨干细胞的最佳来源、最佳给药方案、标准化制备及长期安全性等,以推动干细胞治疗在脓毒症精准医疗方面的应用[42]。
Research progress on stem cells in the treatment of sepsis
doi: 10.12206/j.issn.2097-2024.202509039
- Received Date: 2025-09-23
- Accepted Date: 2026-01-19
- Rev Recd Date: 2025-11-16
- Available Online: 2026-02-12
- Publish Date: 2026-02-25
-
Key words:
- sepsis /
- stem cells /
- immune response /
- inflammation
Abstract: At present, the treatment of sepsis depends largely on non-specific methods, highlighting an urgent need for novel therapeutic strategies. Stem cells have garnered significant attention in the treatment of various diseases due to their unique biological properties. Stem cells enhance sepsis survival through mechanisms such as reducing bacterial burden, modulating inflammation, and ameliorating organ dysfunction. Recent studies have shown that stem cells can increase the survival rate of sepsis patients through multiple pathways such as reducing the bacterial load of the host, regulating inflammatory homeostasis, and improving multi-organ dysfunction. Their derivatives, exosomes, can also alleviate the imbalanced immune response in sepsis patients. Recent advances in stem cell-based therapies for sepsis were summarized in this paper.
| Citation: | CHEN Ting, CHEN Linlin, CHEN Zhao, ZHANG Junping, WANG Yan. Research progress on stem cells in the treatment of sepsis[J]. Journal of Pharmaceutical Practice and Service, 2026, 44(2): 59-64, 70. doi: 10.12206/j.issn.2097-2024.202509039 |







 DownLoad:
DownLoad: