-
腹主动脉瘤(AAA)是一种严重的慢性炎症性退行性主动脉疾病,特征是腹主动脉壁进行性病理性扩张[1-2]。当局部扩张的腹主动脉直径大于正常值的50%时(人体中,最大直径≥30 mm),即可诊断为AAA[3]。大多数AAA患者没有症状,通常缓慢发展,然而也可能迅速进展为大AAA并破裂,死亡率极高[4]。研究表明,每年全球约20万人死于腹主动脉瘤体破裂[5]。高龄、男性、高血压、吸烟、动脉粥样硬化、家族史和环境污染均会增加AAA的患病风险[6-9]。目前大AAA(人体中,>50 mm)的唯一治疗方法就是开放性或血管内动脉修补术,而对于小AAA(人体中,<50 mm),尚无明确可以延缓小AAA进展的药物[5]。因此进一步明确AAA的分子调控机制,寻找潜在的药理学干预靶点至关重要。
α7烟碱乙酰胆碱受体(α7nAChR)是一种由5个相同的α7亚单位组成的配体门控离子通道型受体[10]。作为“胆碱能抗炎通路(CAP)”的核心,参与了机体的急慢性炎症过程[11, 12]。研究表明,激活α7nAChR在多种心脑血管疾病(如心肌缺血再灌注、缺血性脑卒中)中发挥保护作用[13, 14]。主动脉细胞外基质(ECM)降解、血管平滑肌细胞(VSMC)凋亡和血管壁慢性炎症是AAA发病机制的重要病理过程[15-17]。炎症细胞释放的细胞因子不仅会加剧ECM降解,还会导致VSMC细胞凋亡。多种抗炎药物如塞来昔布、c-Jun N-末端激酶抑制剂和缓激肽2受体拮抗剂等均已被证明可以减缓小鼠AAA的进展并降低其破裂的风险[18]。因此抑制炎症反应已被推测是治疗AAA的可行手段[1]。
由此,我们推设激活α7nAChR可通过抑制炎症反应减轻CaCl2诱导的小鼠AAA损伤。本研究通过构建CaCl2诱导的小鼠AAA模型及体外细胞实验来研究激活α7nAChR对AAA的作用及可能机制。
-
α7nAChR基因敲除(KO)和野生型(WT)小鼠由同济大学附属第十人民医院赠送,其饲养与繁育工作于内蒙古医科大学SPF级实验动物室完成。SD大鼠购买自内蒙古医科大学实验动物中心,用于原代主动脉平滑肌细胞(VSMC)的提取。实验进行期间,动物房温度维持在22~25 ℃,湿度保持在70 %左右,且保持7:00至19:00的照明状态。所有动物均能够自由饮水、饮食,且笼内能够循环通风。本实验过程中所有动物操作,均符合内蒙古医科大学动物中心的管理规范。
-
TNF-α抗体(BA0131,Boster);IL-1β抗体(ab205924,abcam);NLRP3抗体(#15101,cst);GSDMD抗体(ab209845,abcam);MMP2抗体(ab92536,abcam);cleaved Caspase 1抗体(#89332,cst);GAPDH抗体,β-actin抗体(碧云天生物技术公司);Donkey anti-mouse荧光二抗,Donkey anti-rabbit 荧光二抗(Li-cor公司);PNU-
282987 ,戊巴比妥钠(Sigma公司);氯化钙(Sigma公司);动物组织DNA抽提试剂盒,琼脂糖,α7nAChR敲除鼠鉴定引物,胶原酶(Ⅱ型)(上海生工生物公司);Taq酶(Takara公司);NaRed 染料(北京派拓科技有限公司);组化试剂盒DAB显色剂(DAKO公司);OCT包埋剂(Sakura公司);EVG染液套装(Service biology公司);蛋白Marker,BCA蛋白测定试剂盒(Thermo Scientific公司);伊红染液,苏木精染液(武汉谷歌生物公司);DAPI染色液(碧云天生物技术公司);PBS缓冲液,50 X TAE溶液(上海博光生物有限公司);FBS,胰蛋白酶(Gibco公司);高糖DMEM培养基(Hyclone公司);TNF-α重组蛋白(Peprotech公司)。 -
Odssey红外荧光显像系统(Li-Cor公司);全自动酶标仪,二氧化碳细胞培养箱(Thermo Scientific公司);包埋机,切片机(武汉俊杰电子有限公司);超净工作台(苏州净化有限公司);台式离心机,普通PCR仪(Eppendorf公司);琼脂糖凝胶电泳仪(Tanon公司);生物电泳图像分析系统(上海复日科技有限公司)。
-
剪取小鼠鼠尾约4 mm,加入组织裂解液,56 ℃水浴1~3 h使鼠尾裂解完全。用动物组织DNA抽提试剂盒进行小鼠DNA的提取。将DNA溶液与Taq酶、α7nAChR的3种引物及水配制成扩增体系,于PCR仪上扩增。
-
称取适量琼脂糖,加入1 XTAE溶液后于微波炉加热使其完全溶解,在冷却之前加入NaRed染料,混匀,倒入配胶装置并插入加样孔梳。待完全冷却后拔出梳子,加样后开始电泳。电泳结束后在生物电泳图像分析系统仪器上成像并拍照分析。
-
7~8周龄的雄性WT小鼠及KO小鼠(α7nAChR−/-)随机分为4组,分别为正常组野生型小鼠(WT)、正常组α7nAChR−/-小鼠(KO)、模型组野生型小鼠(WT+CaCl2)和模型组α7nAChR−/-小鼠(KO+CaCl2)。使用戊巴比妥钠(50 mg/kg,i.p.)麻醉小鼠后,开腹,充分暴露腹主动脉。对肾动脉分支以下至髂总动脉段的腹主动脉进行充分游离,用生理盐水(对照组)或0.5 mol/L的CaCl2溶液(模型组)浸泡后的无菌纱布完全包裹腹主动脉外膜15 min,随后将残留的CaCl2溶液使用生理盐水冲洗,关腹,缝合。术后3 d内于腹部伤口处涂抹青霉素以防感染。28 d后麻醉处死小鼠,获取主动脉标本。小鼠主动脉最大直径扩张1.5倍即判定主动脉瘤形成,即造模成功。
-
固定后的主动脉标本,使用梯度酒精脱水后进行石蜡包埋,切片机连续切片,厚度约4 μm/片。将石蜡切片脱蜡处理后再水化,进行苏木精和伊红染色(HE染色);染色封片后置于显微镜下观察,评估组织学特征。对脱蜡后再水化的切片进行EVG染色以评估主动脉壁的弹性蛋白完整性。
-
将石蜡切片脱蜡处理后与一抗(TNF-α,IL-1β,NLRP3,GSDMD;按照说明书推荐比例配制成相应溶液)于4 ℃孵育过夜,PBS缓冲液洗涤切片,随后加入相应二抗孵育1 h,再次用PBS缓冲液洗涤后用DAPI对细胞核进行染色。染色结束后将切片置于显微镜下观察并评估抗体表达水平。
-
SD大鼠麻醉后开腹获取主动脉。转移至超净台,用胶原酶溶液于37 ℃细胞培养箱中消化30 min,取出主动脉用PBS缓冲液清洗后置于含20 %FBS的高糖DMEM培养基中,于37 ℃细胞培养箱中培养24 h。随后用PBS缓冲液清洗后将其置于含有胶原酶和胰蛋白酶的的混合消化液中,37 ℃反应2 h。终止消化后收集组织细胞悬液,离心弃上清液,加入含20%FBS的高糖DMEM培养基重悬细胞,置于37 ℃继续培养,2~3 d后换液并传代。选用第3~8代细胞用于后续实验。
-
探究TNF-α刺激VSMC不同时间点(0、3、6、12、24、48 h)的炎症因子表达,分为6组,分别为对照组(Con,即TNF-α刺激细胞0 h),3 h处理组(3 h),6 h处理组(6 h),12 h处理组(12 h),24 h处理组(24 h)和48 h处理组(48 h)。探究PNU-282987激活α7nAChR对VSMC炎症因子表达的影响,分为对照组(Con),模型组(TNF-α,TNF)和模型加药组(TNF-α+PNU-282987,TNF + PNU)。
-
取各组处理后VSMC细胞,采用BCA法测定蛋白浓度。蛋白经SDS-PAGE分离后转移至PVDF膜,封闭后与一抗NLRP3(#15101,cst,USA),MMP2(ab209845,abcam公司,UK)于4 ℃孵育过夜,洗涤后加入荧光二抗孵育液,室温避光孵育2 h后重复洗涤3次。用Odyssey红外成像系统成像并拍照,结合Quantity One软件分析蛋白表达水平,以β-actin或GAPDH为内参。
-
计量资料以均数±标准误(means±SEM)表示。利用GraphPad Prism 8.0 软件进行统计分析,两组间比较采用非配对T检验,多组之间选用单因素方差分析。P<0.05表示差异有统计学意义。
-
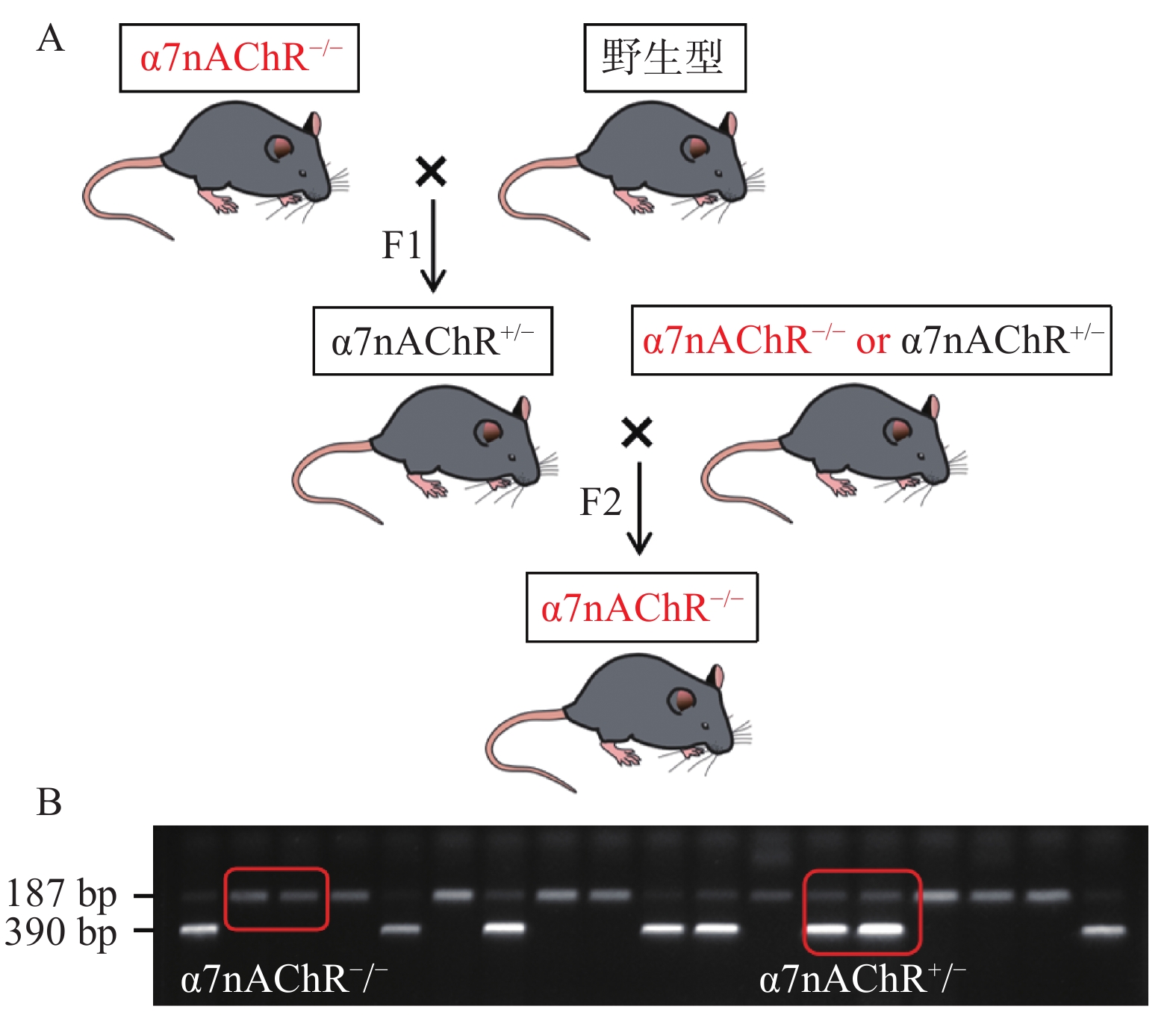
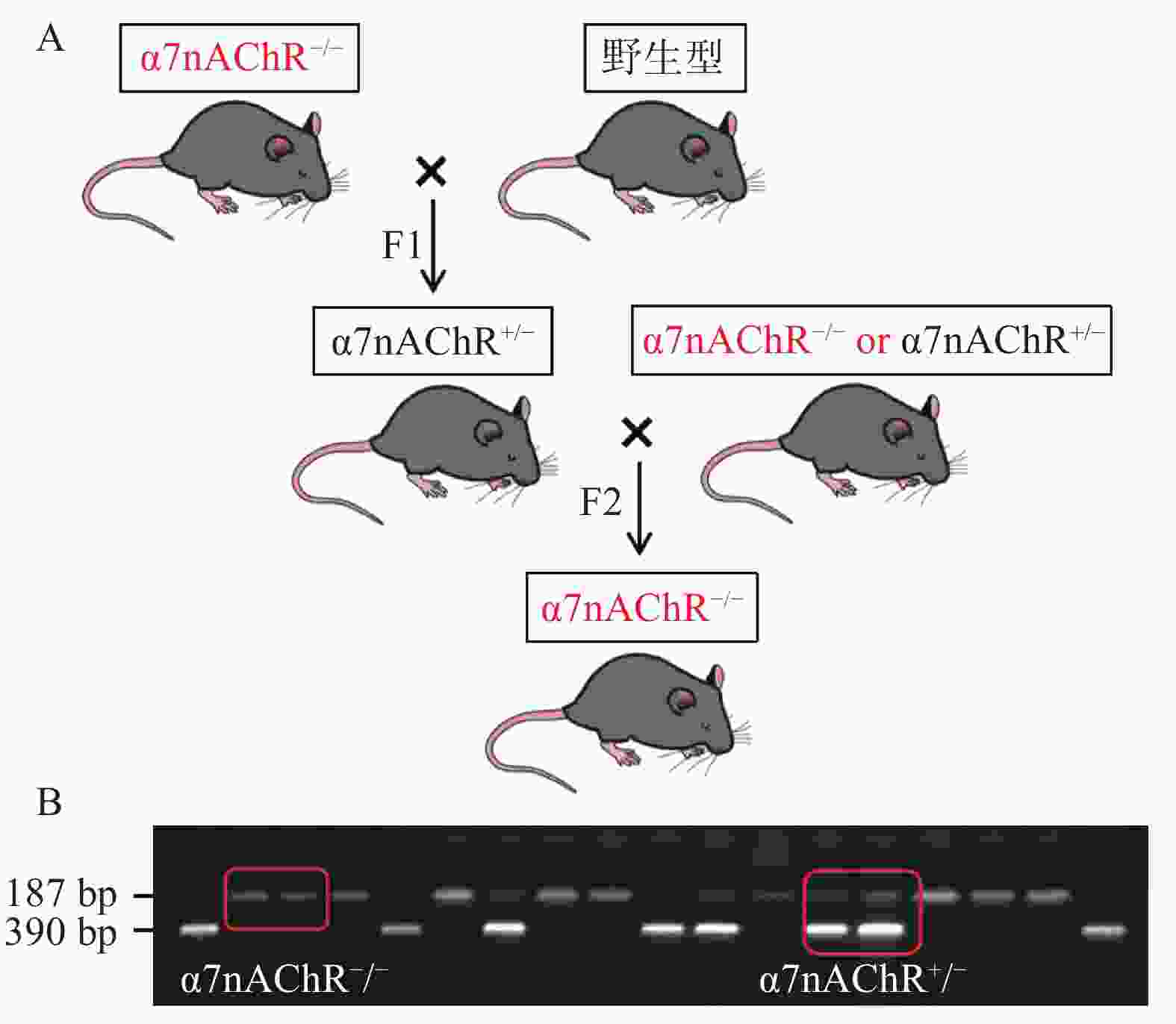
根据以下小鼠繁育模式,我们将α7nAChR敲除(α7nAChR−/-)小鼠与野生型(WT)小鼠配种,可得到α7nAChR−/-小鼠、α7nAChR+/-小鼠、WT小鼠;通过获取小鼠尾部DNA并进行琼脂糖凝胶电泳,判定子代小鼠基因型。若在小分子量处出现一条带(187 bp),则为α7nAChR−/-小鼠;若在大分子量处出现一条带(390 bp),则为野生型小鼠(WT);若有两个条带(390 bp和187 bp)出现,则为α7nAChR+/-小鼠。
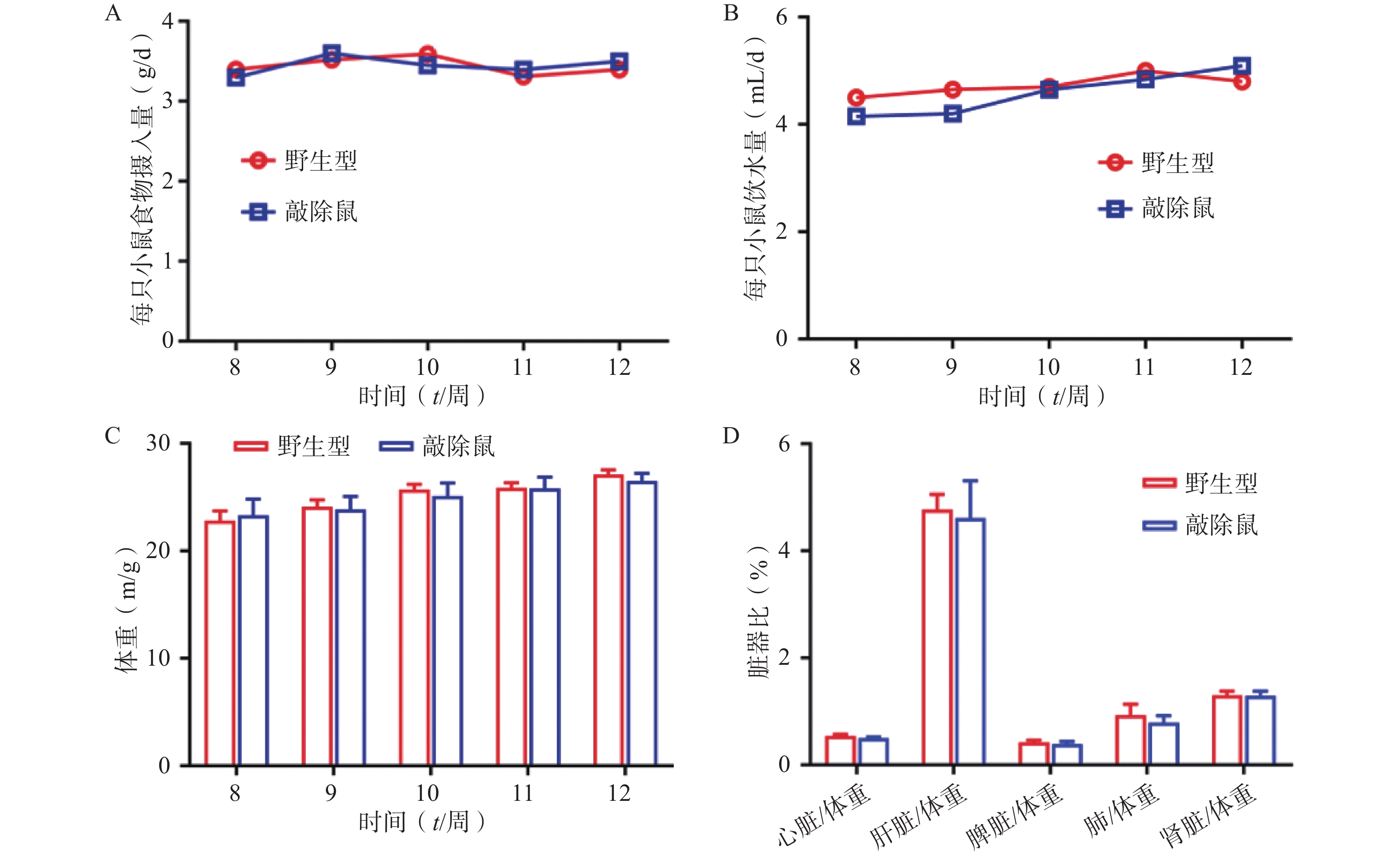
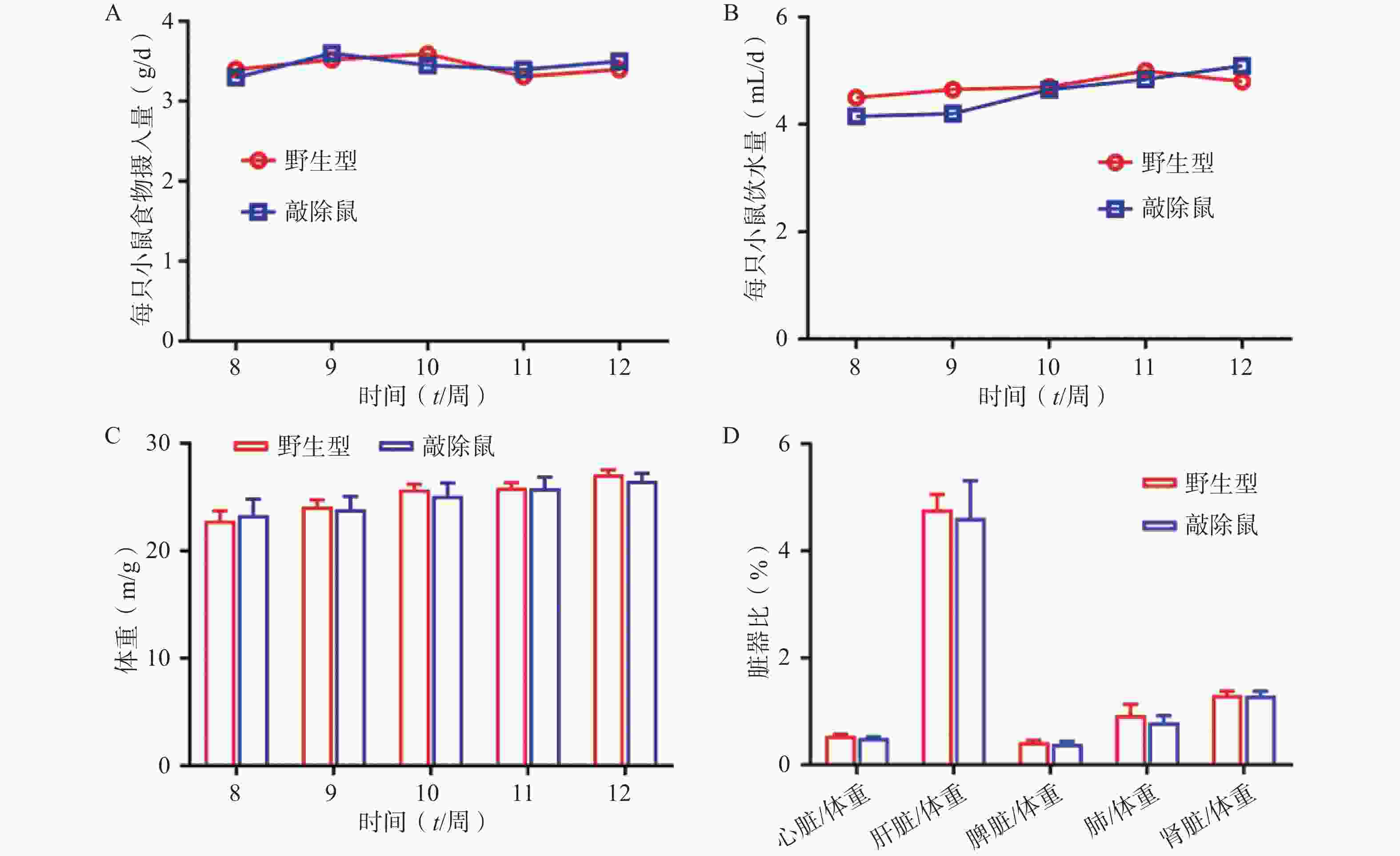
为明确敲除α7nAChR对小鼠基本生理指标是否有影响,我们同步记录α7nAChR−/-小鼠和WT小鼠(8~12 周龄)的进食、饮水量及体重变化;同时选用8周龄α7nAChR−/-小鼠与WT小鼠,检测两者心脏、肝脏、脾脏、肾脏、肺与体重的比值。结果发现:两组小鼠的体重、进食饮水量及重要脏器体重比均无显著差异。以上结果提示敲除α7nAChR对小鼠基本生理体征无明显影响。
-
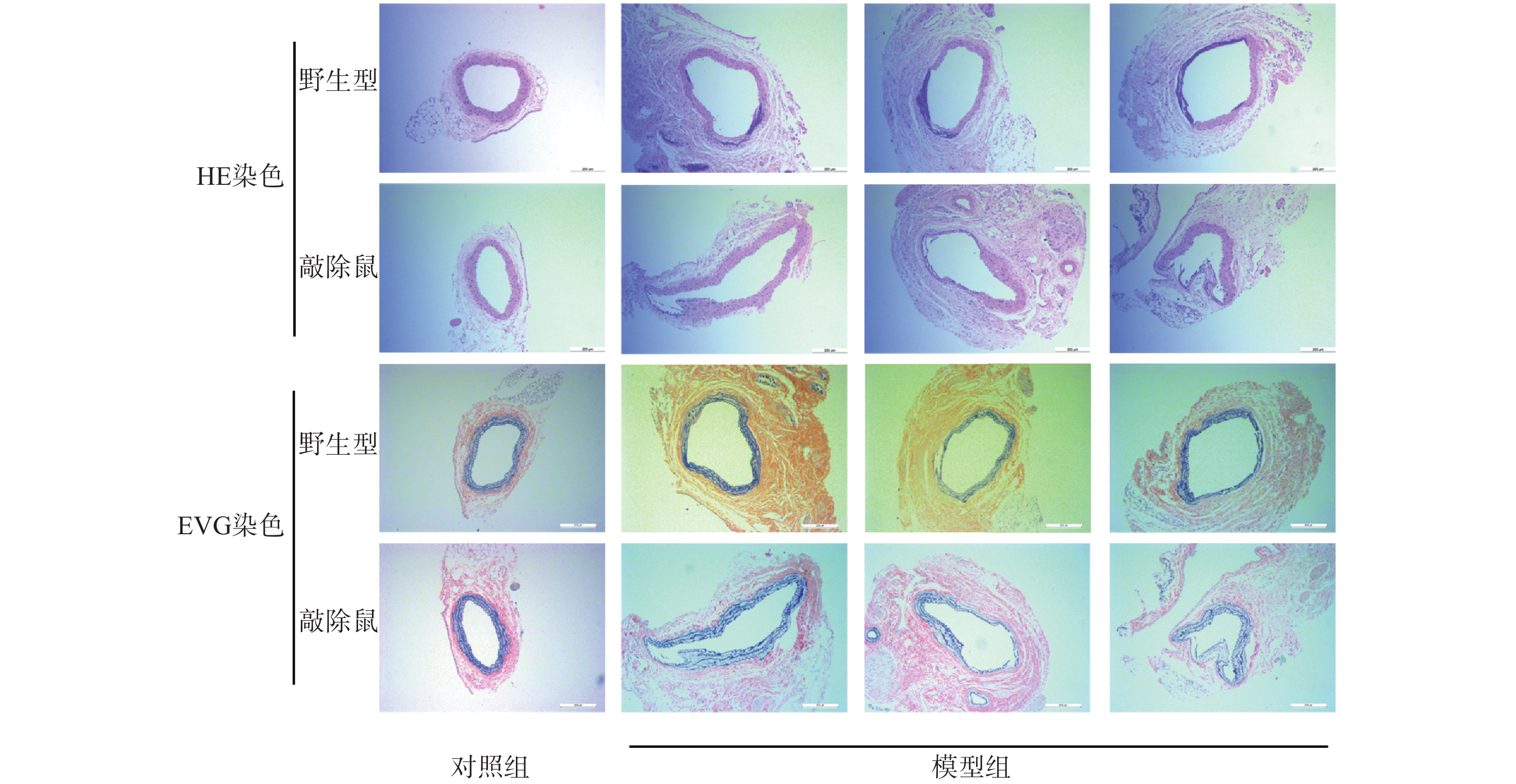
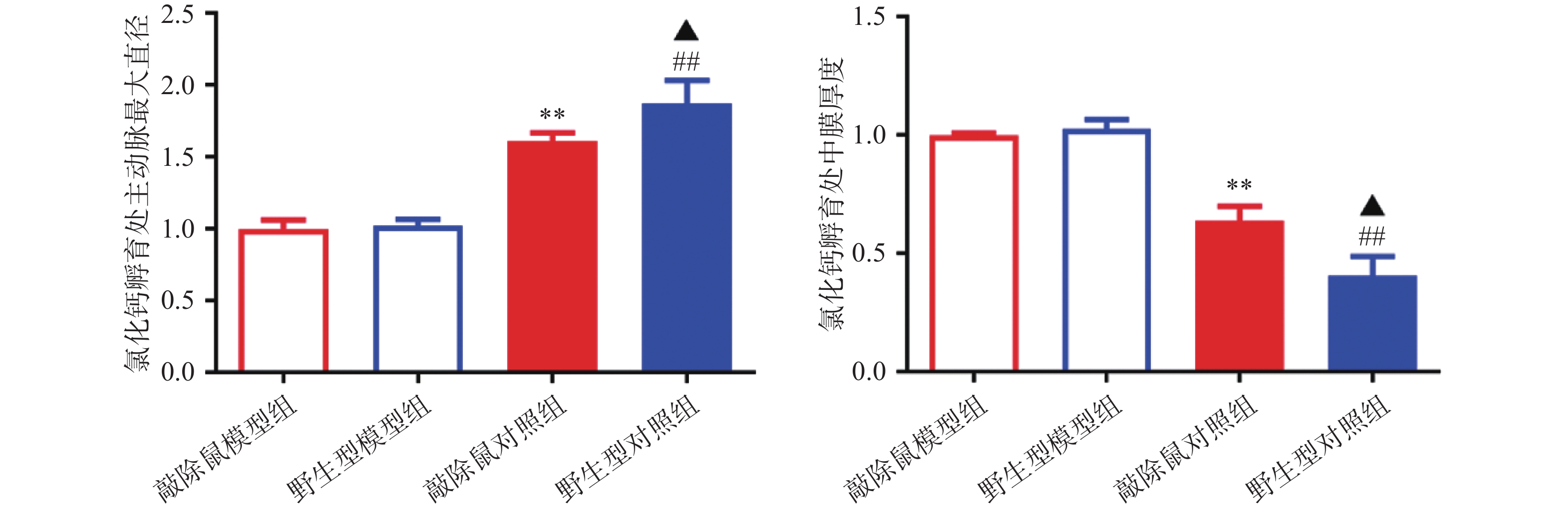
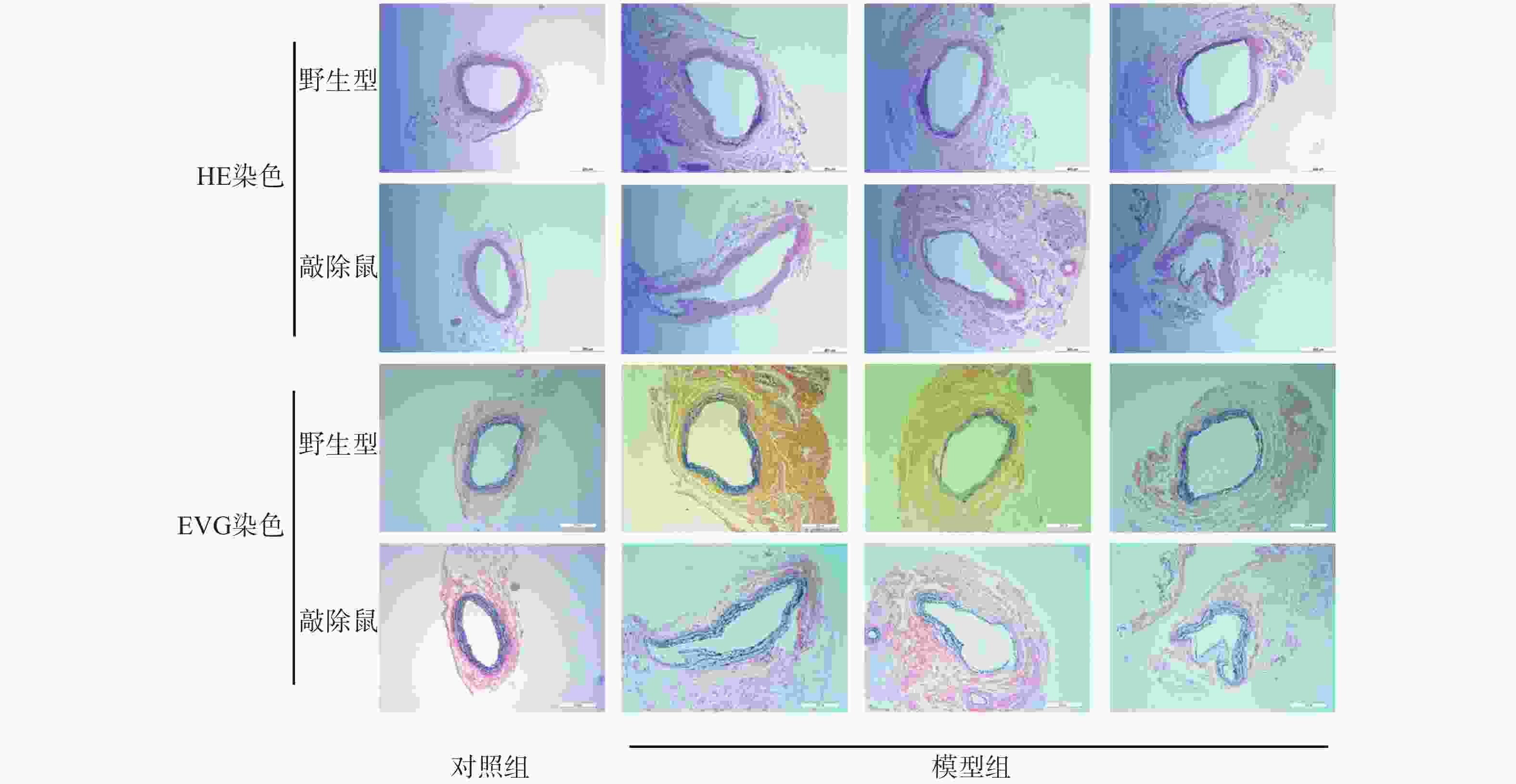
与其他啮齿类动物AAA模型相比,CaCl2诱导的AAA小鼠模型病变部位常位于肾动脉分支下段的腹主动脉,与人类AAA相似[19]。故我们选用CaCl2诱导小鼠形成AAA模型。如图所示,对照组的WT与KO小鼠主动脉壁的3层组织结构即内膜、中膜和外膜均清晰可见,弹性纤维结构完整,走形规则,厚度均匀。与对照组相比,CaCl2孵育后的AAA小鼠主动脉壁组成结构明显被破坏,中膜的弹性纤维层显著变薄,甚至断裂、消失,中膜部分僵直并失去正常的波浪状结构,有的呈烧焦状。值得注意的是,敲除α7nAChR的小鼠CaCl2孵育后其主动脉的损伤更为严重。
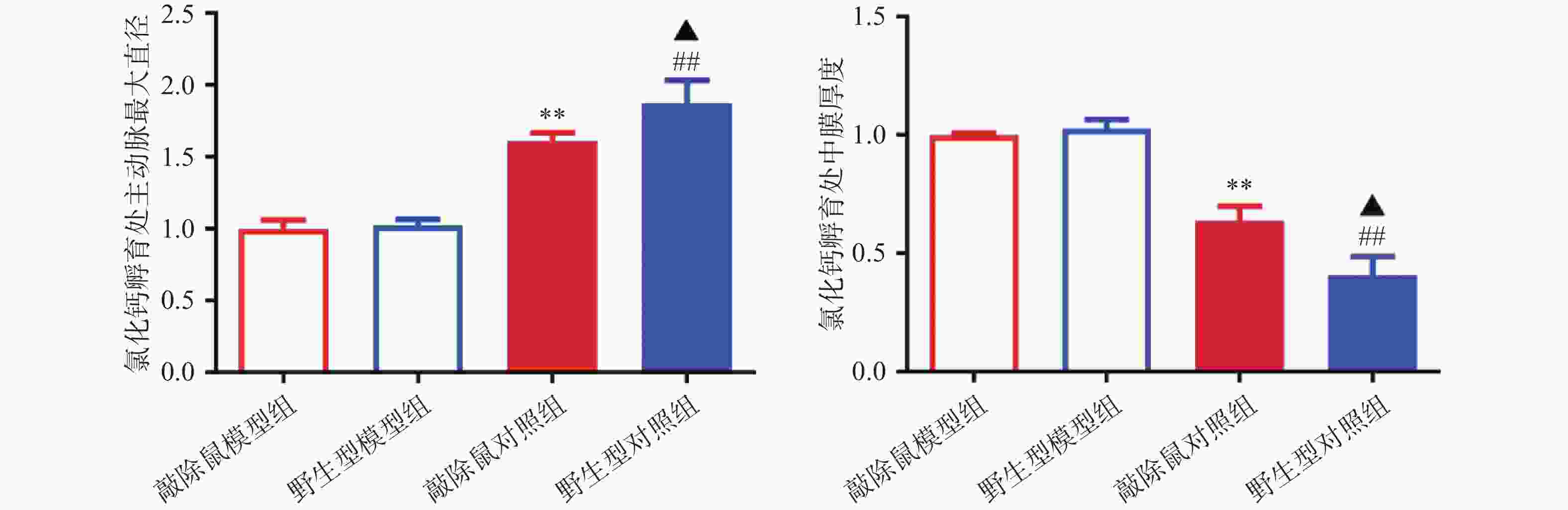
对HE染色结果进行统计分析发现:WT和KO对照组小鼠的主动脉直径及中膜厚度,无明显差异;CaCl2孵育后,模型组小鼠主动脉明显扩张,中膜明显变薄,证明我们成功构建小鼠AAA模型;与WT小鼠AAA组相比,KO小鼠主动脉进一步扩张,中膜厚度进一步减小,即KO小鼠AAA模型中腹主动脉损伤更严重。
-
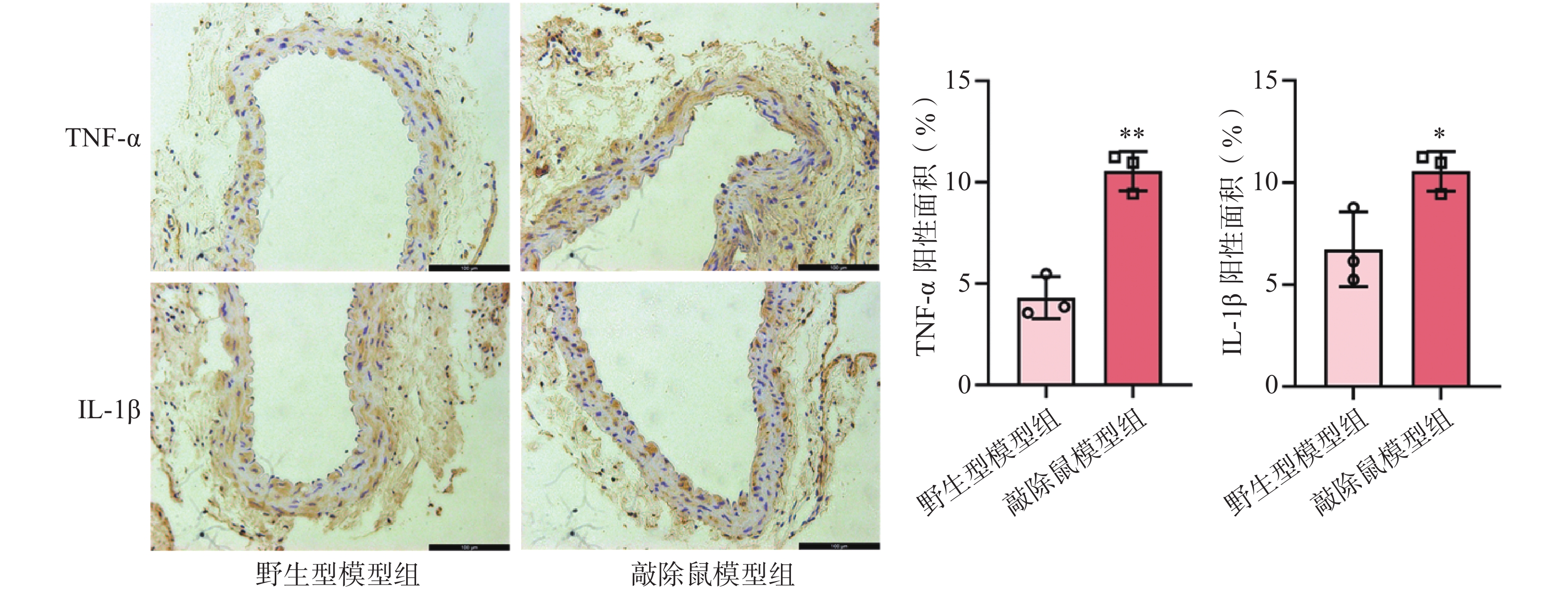
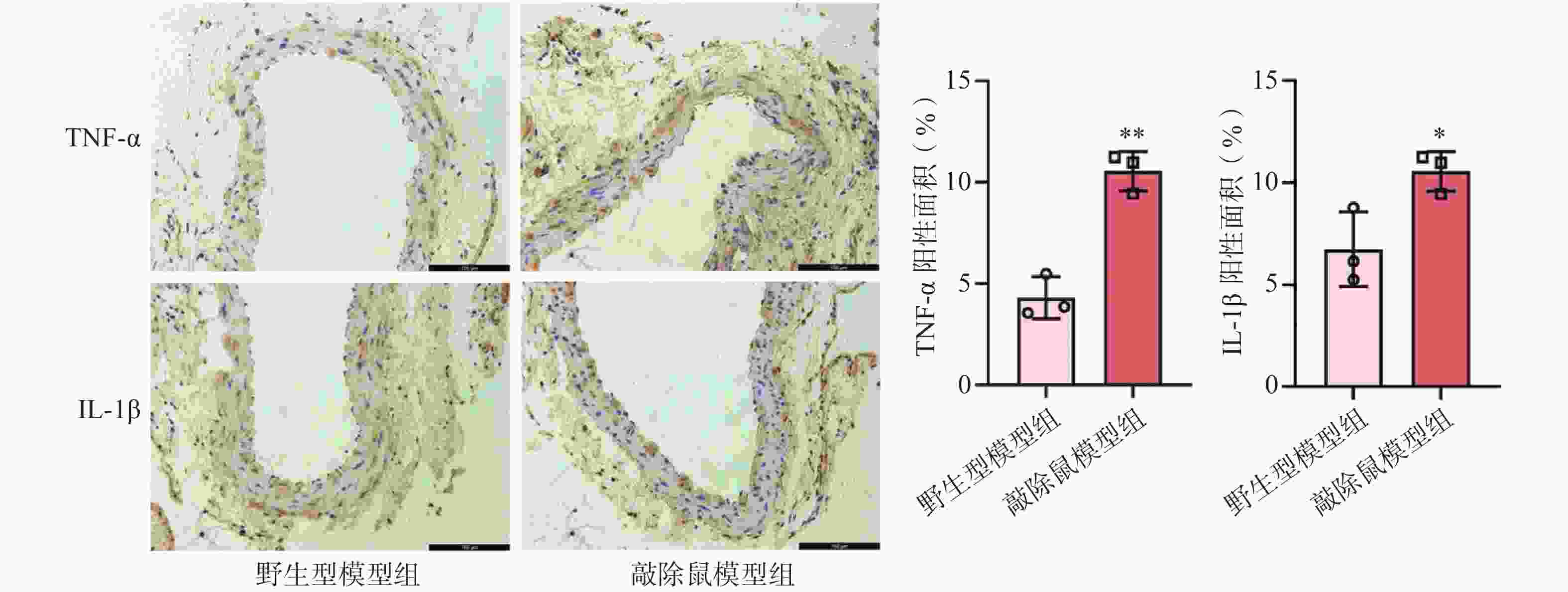
研究认为,血管慢性炎症是腹主动脉瘤形成的核心因素之一[20]。我们前面的研究证明敲除α7nAChR明显加重了AAA小鼠的主动脉损伤。为了验证这一结果是否与加重小鼠血管壁炎症反应相关,我们通过免疫组化染色检测了各组小鼠主动脉中炎症因子的表达情况。结果显示,CaCl2诱导后,与WT小鼠相比,敲除α7nAChR显著增加了AAA小鼠主动脉中炎症因子TNF-α及IL-1β的表达。
-
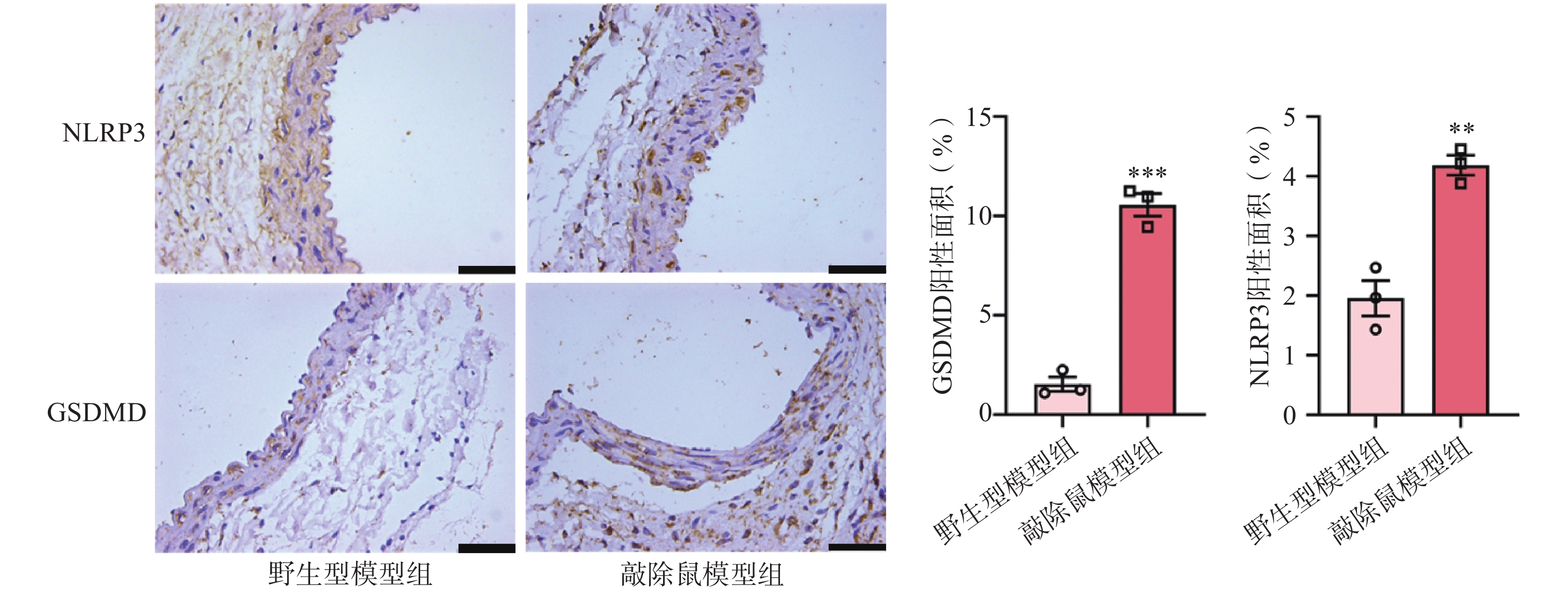
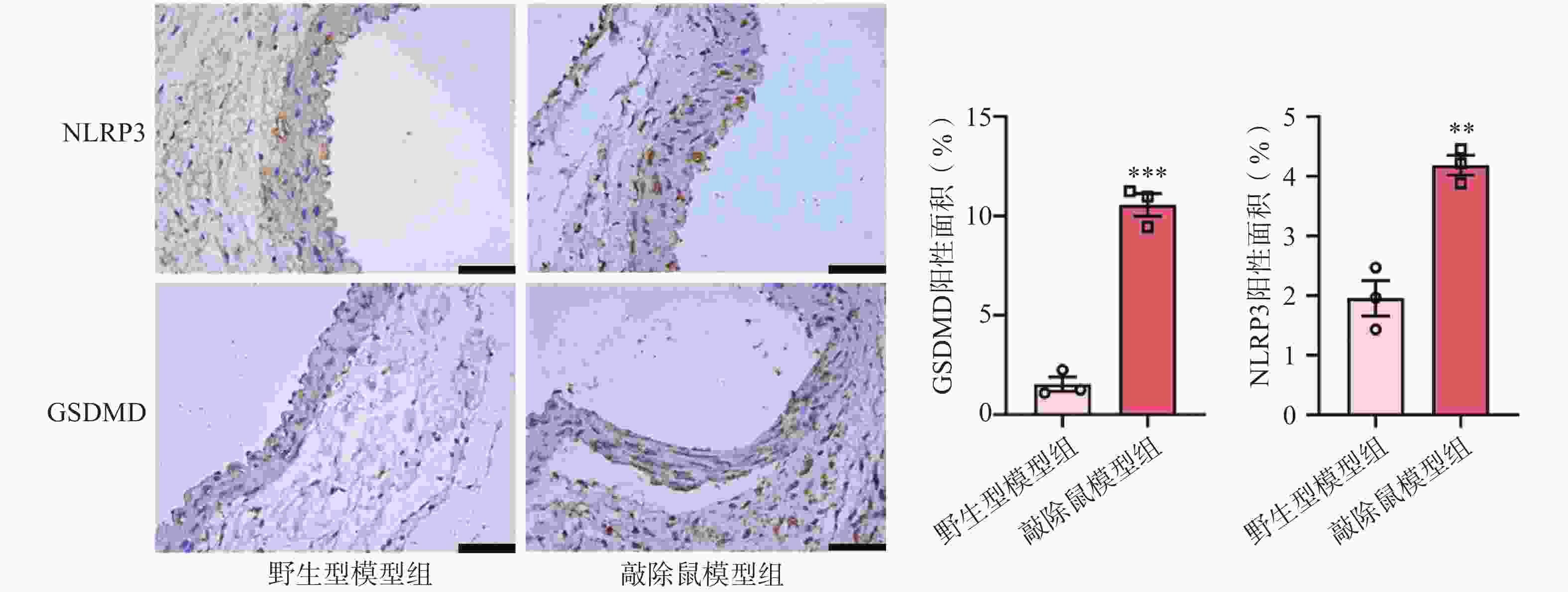
近来,许多研究表明NLRP3炎症小体与AAA的发生发展有一定相关性[21-22]。NLRP3的激活可以促进pro-Caspase-1的自我剪切,GSDMD可以被激活的Caspase-1切割,N-GSDMD通过在细胞膜上形成寡聚孔从而引发细胞焦亡[23]。因此,我们评估了NLRP3和GSDMD在AAA中的表达。免疫组化染色结果显示,与对照组AAA小鼠相比,小鼠缺失α7nAChR后,其主动脉中NLRP3和GSDMD的表达水平明显上调。这一结果提示敲除α7nAChR使AAA小鼠主动脉损伤更严重可能是通过激活NLRP3及下游GSDMD进一步促进炎症因子释放所致。
-
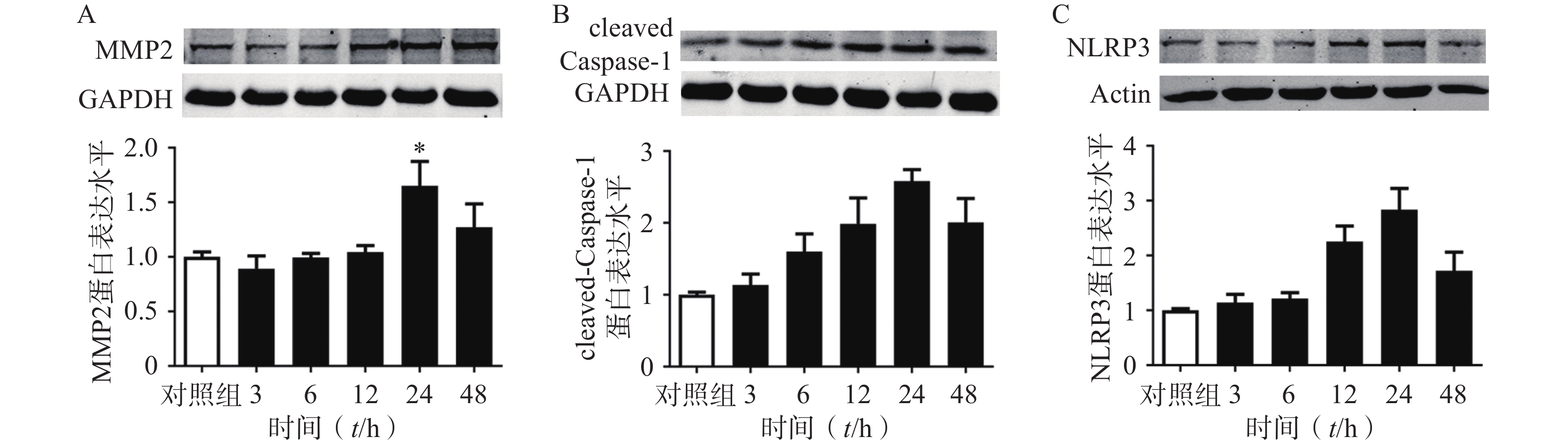
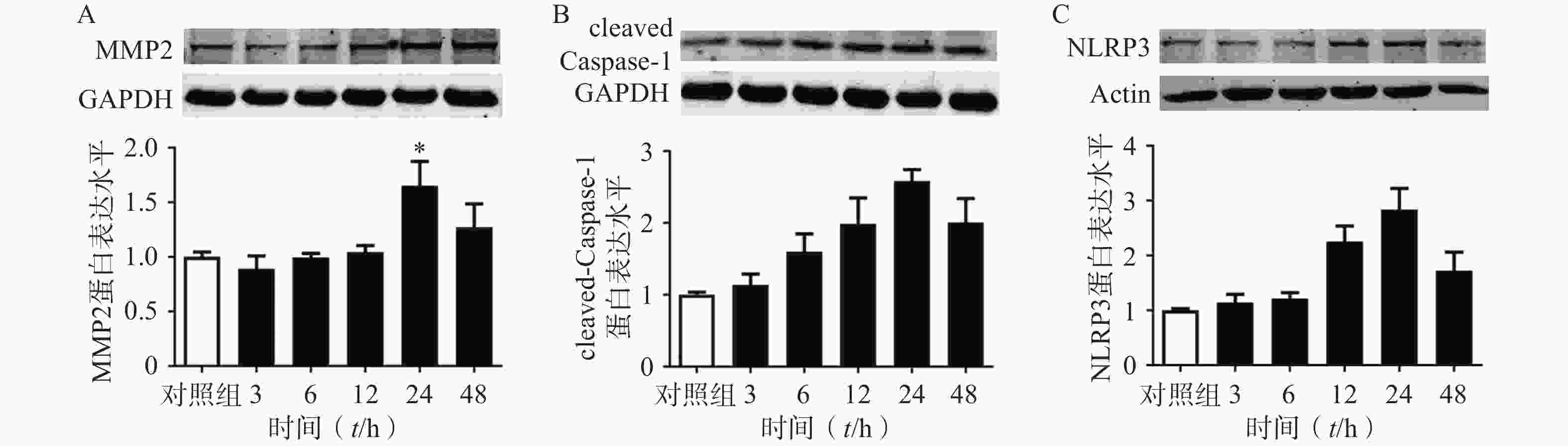
选用100 ng/ml的TNF-α刺激大鼠原代VSMC细胞以模拟AAA血管慢性炎症的微环境[24]。首先我们在大鼠来源的原代VSMC上,明确了TNF-α刺激细胞不同时间对基质金属蛋白酶2(MMP2),cleaved Caspase1,NLRP3蛋白表达的影响。结果表明:随着TNF-α刺激VSMC时间的增加,MMP2,cleaved Caspase1,NLRP3的蛋白表达逐渐增多,在24 h时达到峰值。因此我们后续实验选择的TNF-α给药时间点为24 h。
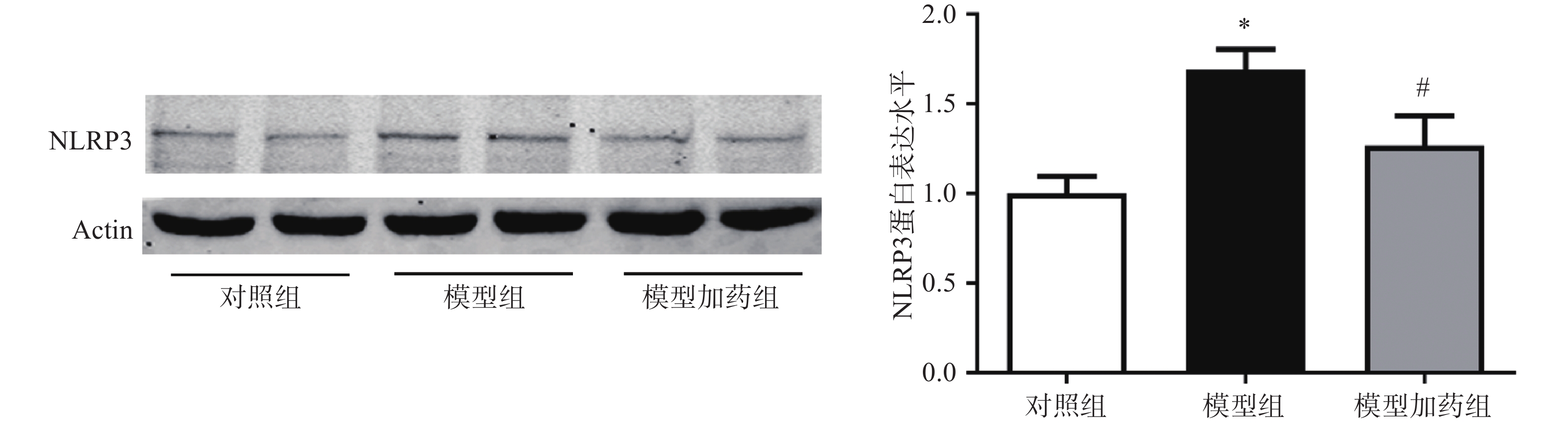
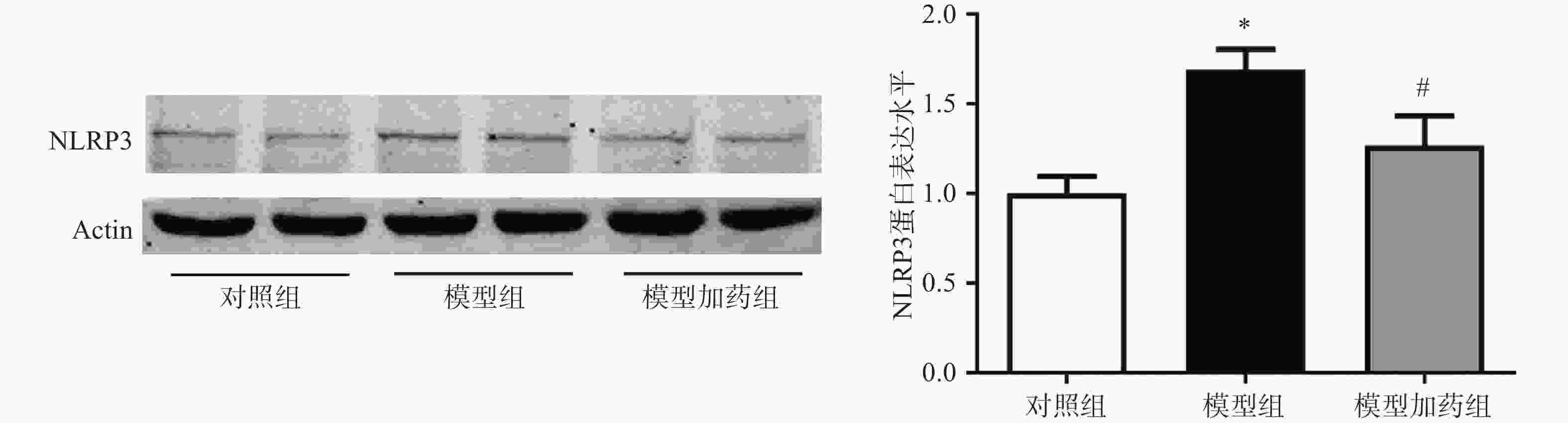
上述体内实验已证明敲除α7nAChR可能通过激活NLRP3炎症小体进一步促进炎症因子的释放而加重AAA小鼠主动脉损伤。因此我们猜测,激活α7nAChR能够抑制NLRP3炎症小体的激活。通过western-Blot检测PNU-
282987 (10 µmol/L)激活α7nAChR对NLRP3表达的影响。结果显示,与对照组相比,TNF-α模型组NLRP3的表达明显上调,与AAA小鼠中NLRP3表达增加一致;激活α7nAChR能够显著抑制TNF-α诱导的NLRP3表达上调。 -
腹主动脉瘤(AAA)一旦破裂,其致死率高达80 %以上[25]。近年来,我国逐步迈入老龄化社会,随着人口老龄化的加剧,AAA的患病率也逐年增加[26]。因此探寻减慢AAA扩张、延缓AAA病理进程的药理学干预靶点迫在眉睫。
血管壁慢性炎症在AAA发生发展过程中发挥着十分重要的作用。不论是在动物AAA模型还是人体病变的AAA组织中,均能检测到炎症细胞的浸润,如CD4+T细胞和巨噬细胞[26]。Ang Ⅱ诱导ApoE−/-小鼠形成AAA模型是目前应用最广泛的造模方式,但该模型更接近主动脉夹层的病理特征;且瘤体多发生在肾动脉分支以上部位的主动脉处,与人类不同[19]。而CaCl2诱导的小鼠AAA模型的病变部位常位于肾动脉分支下段的腹主动脉,正是人类AAA的常见部位;且该模型与人类AAA疾病相似的病理特征包括主动脉组织钙化、平滑肌细胞凋亡、蛋白酶活性增强、弹性蛋白被吸收、中膜变薄等[20]。因此,我们选用CaCl2诱导小鼠AAA模型来探究α7nAChR在AAA中的作用。结果表明,CaCl2孵育成功诱导小鼠AAA模型,且与WT小鼠AAA组相比,α7nAChR敲除鼠AAA组的主动脉损伤更为严重,α7nAChR的缺失也进一步加重了AAA小鼠的炎症反应。
最近的研究报道,α7nAChR可通过抑制小胶质细胞的NLRP3炎症小体对小鼠缺血性脑卒中起保护作用[13];MCC950通过阻断巨噬细胞中NLRP3炎症小体激活而抑制小鼠腹主动脉瘤的形成[27]。众所周知,NLRP3炎症小体的激活可以促进pro-Caspase-1的自我剪切,GSDMD可以被活化的Caspase-1切割,N-GSDMD通过在细胞膜上形成寡聚孔促使炎症因子释放从而引发细胞焦亡[23]。我们猜测NLRP3炎症小体的激活参与了AAA的病理生理进程,并评估了NLRP3及GSDMD在AAA中的表达。研究发现,敲除α7nAChR显著增加了AAA中NLRP3及GSDMD蛋白的表达;激活α7nAChR可抑制TNF-α诱导的VSMC中NLRP3表达的上调。
综上所述,敲除α7nAChR促使炎症因子增多加重CaCl2诱导的小鼠AAA损伤,而激活α7nAChR可减少炎症因子释放减轻小鼠AAA损伤,其机制可能与调控NLRP3/GSDMD通路相关。本实验对激活α7nAChR减轻CaCl2诱导小鼠AAA损伤的可能机制进行了初步探索,相关作用机制仍需更深入的研究。
Amelioration chloride-induced abdominal aortic aneurysm injury by activation of α7nAChR s calcium in mice
doi: 10.12206/j.issn.2097-2024.202409045
- Received Date: 2024-09-20
- Rev Recd Date: 2025-04-16
-
Key words:
- α7nAChR /
- inflammation /
- CaCl2 /
- abdominal aortic aneurysm
Abstract:
| Citation: | ZHANG Wenjing, FU Hui, GUO Xiaobin, GUO Hao. Amelioration chloride-induced abdominal aortic aneurysm injury by activation of α7nAChR s calcium in mice[J]. Journal of Pharmaceutical Practice and Service. doi: 10.12206/j.issn.2097-2024.202409045 |







 DownLoad:
DownLoad: