-
阿尔茨海默病(AD)是一种以进行性记忆功能和神经行为障碍为表现的中枢神经系统退行性疾病。目前全球约有AD患者
5000 余万人,随着人口老龄化的进展,这一数字还将持续增加,给全球发展带来巨大的健康和经济负担[1] 。到目前为止,临床尚缺乏有效的AD治疗手段。胆碱酯酶抑制剂、NMDA拮抗剂等传统AD治疗药物效果有限,FDA新批准上市的Aβ单克隆抗体仑卡奈单抗等疗效尚存争议,且治疗费用昂贵[2] 。因此,开发经济、有效的AD治疗药物仍是当前研究热点。中药因其多靶点系统作用和低毒副作用的优势,近年来在AD等复杂疾病治疗药物发掘中发挥重要作用[3] 。中药葛根和知母临床应用历史悠久,葛根解肌退热、生津止渴,知母清热泻火、滋阴润燥,二者配伍可清热生津、滋阴润燥,改善代谢紊乱,对热邪灼津、痰浊阻窍所致的健忘呆钝、消渴等症具有治疗作用,主要代表方剂为玉液汤[4-6] 。近年来研究发现该药对的一些成分如葛根素[7] 、芒果苷[8] 、知母皂苷BⅡ[9] 等对AD具有药效作用。作为一种复杂的异质性疾病,AD的发生与糖尿病存在紧密的因果关联,也被称为脑型糖尿病(3型糖尿病)[10] 。但是目前鲜见葛根与知母配伍后在AD治疗中作用效果的报道。因此,本研究拟通过建立AD大鼠模型考察葛根和知母配伍防治AD的效果,同时运用代谢组学策略探究葛根与知母作为药对配伍后防治AD潜在的作用机制,为中药防治AD研究提供参考借鉴。
-
Agilent
1290 Infinity液相色谱仪和6538 UHD Accurate-Mass 四级杆飞行时间串联高分辨质谱仪(美国安捷伦公司);HERAEUS FRESCO 17高速冷冻离心机(美国赛默飞公司);ANALOG 涡旋振荡器(美国奥豪斯公司);Milli-Q Integral超纯水机(美国Millipore公司);十万分之一电子分析天平(日本A&D公司);Digbehav动物行为学分析系统-水迷宫(上海吉量软件科技有限公司)。 -
葛根(批号:A220901)与知母药材饮片(批号:
20220201 )购自上海市白鹿堂中药店,经海军军医大学药学系蒋益萍副教授鉴定为豆科植物野葛P. lobate(Willd.)Ohwi的干燥根和百合科植物知母A. asphodeloides Bge.的干燥根茎;乙醇(分析纯,国药集团上海化学试剂有限公司); 乙腈、甲酸(均为LC-MS级,赛默飞世尔科技中国有限公司);乙腈、甲醇(色谱纯,北京迪马科技有限公司);乌拉坦(批号:P2091859)、D-gal(批号:P1616089)和AlCl3(批号:P2391168)购自上海泰坦科技股份有限公司;生理氯化钠溶液(四川科伦药业股份有限公司);L-2-氯苯丙氨酸(98%,上海麦克林生化有限公司);超氧化物歧化酶(SOD)、丙二醛(MDA)和一氧化氮(NO)试剂盒购自上海源桔生物科技中心。 -
健康雄性清洁级SD大鼠,体重(200±20)g,购自浙江省实验动物中心,动物生产许可证号:SCXK(浙)2019-0002。大鼠饲养于海军军医大学药学系实验动物中心,笼饲条件为:温度恒定为(22±2)℃,湿度区间为40%~60%,昼夜循环时间为12 h。
-
分别取葛根、知母和葛根-知母药对(15∶12)粉末适量,以10倍体积70%乙醇浸泡,在85 ℃下加热回流提取90 min,滤过,滤渣以同等条件重复提取2次。合并3次滤液,60 ℃减压浓缩至无乙醇味,制备得到用于灌胃的提取液,密封保存于−20 ℃待用。
-
40只SD大鼠适应性喂养1周后按照体重随机分为空白对照组、AD模型组、葛根组、知母组和葛根-知母药对组共5组(n=8)。除对照组外,其余4组大鼠每日给予D-gal 300 mg/kg腹腔注射和AlCl3 200 mg/kg灌胃,连续给药21周建立AD动物模型。对照组每日给予等量的生理盐水(灌胃+腹腔注射)。自第14周起,3个中药干预组分别给予葛根、知母和葛根-知母药对提取液灌胃(相当于生药量:葛根6.25 g/(kg·d),知母5 g/(kg·d),药对11.25 g/(kg·d)。对照组和模型组大鼠灌胃等量纯水。
-
采用Morris水迷宫行为学实验评价大鼠的学习和记忆能力。水迷宫实验全程共6 d,其中包括5 d的定位航行训练和1 d的空间探索试验。利用动物行为学分析系统记录大鼠在定位航行训练期间每日的逃避潜伏时间和空间探索训练中的运动轨迹、穿越站台次数、各象限的运动距离和停留时间等参数,供分析评价使用。
-
行为学实验结束后,大鼠腹腔注射乌拉坦麻醉,经腹主动脉取血,静置后,在4 ℃、
4 000 r/min转速下离心10 min,取上清液分装冻存于−80 ℃,供后续分析用。 -
使用ELISA试剂盒,按照说明书步骤检测大鼠血清中的SOD、MDA、NO等氧化应激和脂质过氧化相关指标。
-
精密称取L-2-氯苯丙氨酸适量,加入甲醇溶解得浓度为5 mg/ml的内标母液,随后用甲醇稀释得到浓度为2 μg/ml的内标溶液。
-
各取200 μl解冻后的血清样本置于1.5 ml的离心管中,加入600 μl预冷的含内标的甲醇溶液,涡旋2 min后,在4 ℃、
12 500 r/min转速下离心15 min,取上清液供UPLC-Q/TOF-MS分析用。取各样本20 μl,混合得到质控(QC)样本。 -
色谱条件:反相色谱柱为Waters X Select HSS T3柱(2.1 mm×100 mm, 1.8 μm),亲水作用色谱柱为Waters Acquity UPLC BEH HILIC柱(2.1 mm×100 mm, 1.8 μm);进样量:2 μl;柱温:40 ℃;流动相为0.1%甲酸-水(A )和0.1%甲酸-乙腈溶液(B);T3柱梯度洗脱模式:0~2 min,2%B;2~17 min,2%~98% B;17~19 min,98%B。HILIC柱梯度洗脱模式:0~2 min,95%B;2~4 min,95%~89% B;4~10 min, 89% B;10~12 min, 89%~66% B;12~15 min, 66% B。流速:0.4 ml/min;色谱柱平衡时间:5 min。
质谱条件:采用 ESI 离子源,正、负离子检测模式;干燥气温度,350 ℃,干燥气体流量:11 L/min;毛细管电压:正离子模式为
4 000 V,负离子模式为3 500 V;碎裂电压:120 V;质谱扫描范围:50~1 500 m/z。 -
质谱数据用XCMS程序包进行预处理,按80%原则过滤无效数据并进行内标归一化处理。使用SIMCA 14.1(Umetrics公司,瑞典)进行偏最小二乘判别分析(PLS-DA)和正交矫正偏最小二乘判别分析(OPLS-DA)并进行模型验证,结合R2X、R2Y和Q2判断模型的拟合效果和预测效果。以变量权重值(VIP)>1、P<0.05且差异倍数(fold change,FC)>1.2或<0.8作为筛选标准,获得AD疾病关联生物标志物。借助HMDB数据库(https://hmdb.ca/)等在线代谢物质谱数据库对筛选得到的差异代谢物进行比对和注释。借助MetaboAnalyst 6.0(https://www.metaboanalyst.ca/)网站进行代谢通路分析。
-
使用SPSS Statistics 23(IBM公司,美国)和GraphPad Prism 8(Graphpad软件公司,美国)进行统计分析与绘图。两组间比较采用t检验,多组间比较采用单因素方差分析,P<0.05认为组间差异具有统计学意义。
-
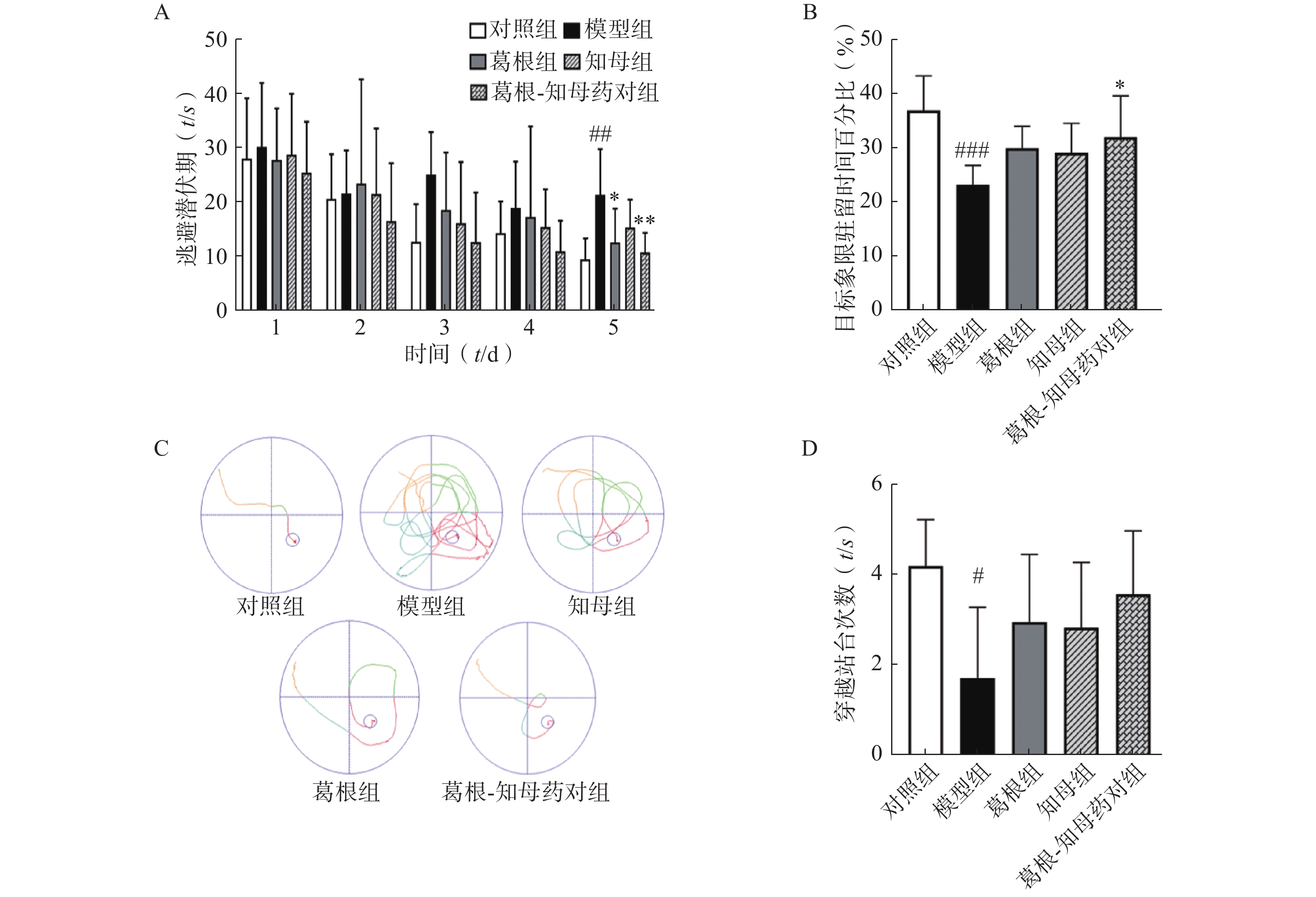
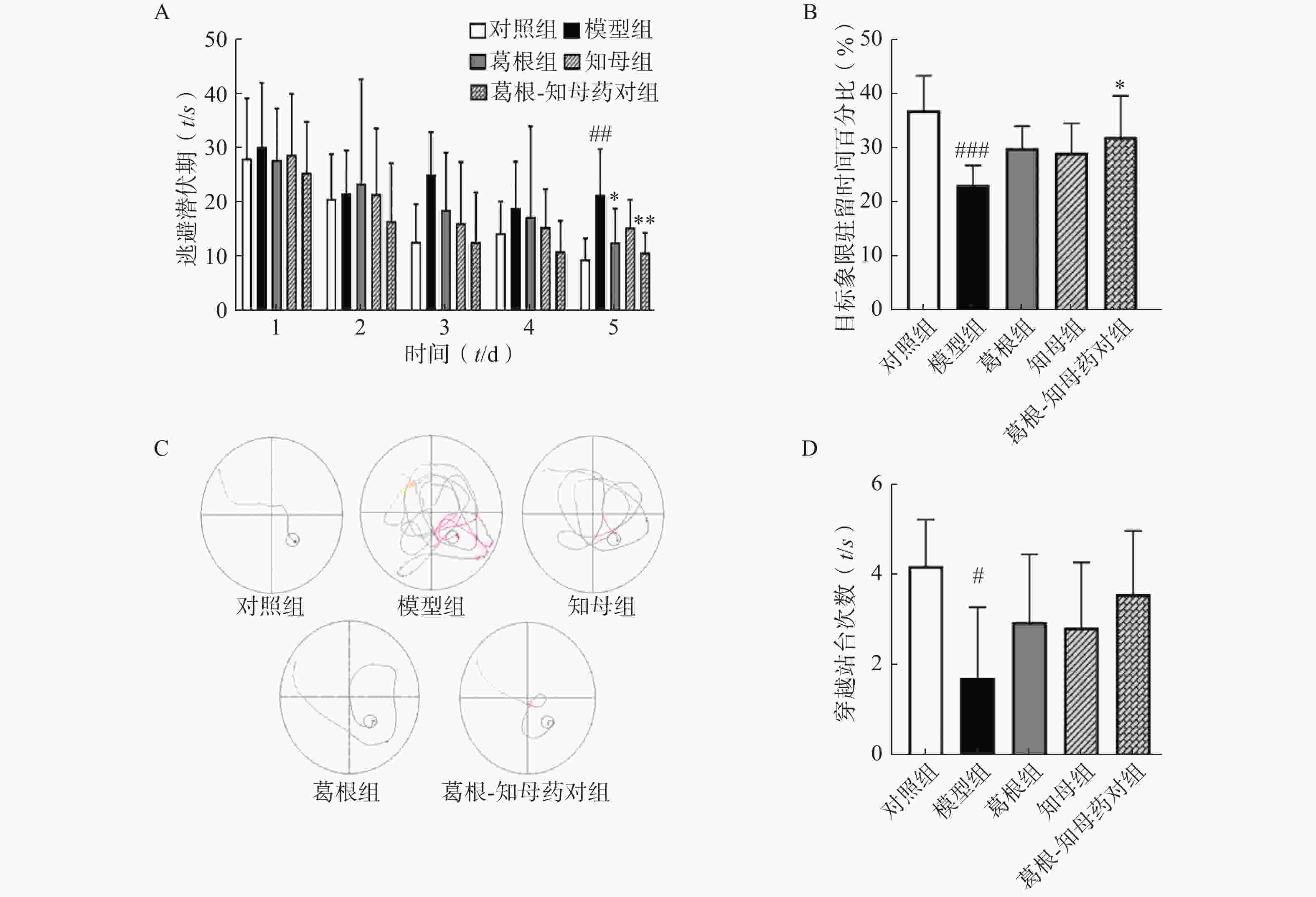
以水迷宫实验中大鼠逃避潜伏期、穿越站台所在位置次数以及站台所在象限的停留时间作为评价指标,考察大鼠的学习和记忆水平。结果如图1所示,定位航行训练期间,各组大鼠的逃避潜伏期随训练时间增加均呈下降趋势,其中模型组逃避潜伏期下降趋势较为平缓,对照组和3个中药干预组下降趋势均较模型组显著,对照组和葛根-知母药对组第5日逃避潜伏期较模型组均有极显著差异(P<0.01)。同样,空间探索实验中,模型组大鼠穿越站台次数以及站台所在象限的停留时间较对照组均显著减少,组间差异具有统计学意义(P<0.05)。中药干预后各组大鼠穿越站台次数及目标象限停留时间均有所增加,其中,葛根-知母药对组与模型组间差异具有统计学意义(P<0.05)。结果表明,造模后大鼠的学习和记忆能力出现下降,给予葛根、知母和葛根-知母药对干预均可不同程度改善大鼠的学习和记忆能力,以葛根-知母药对最为显著,效果优于单药。
-
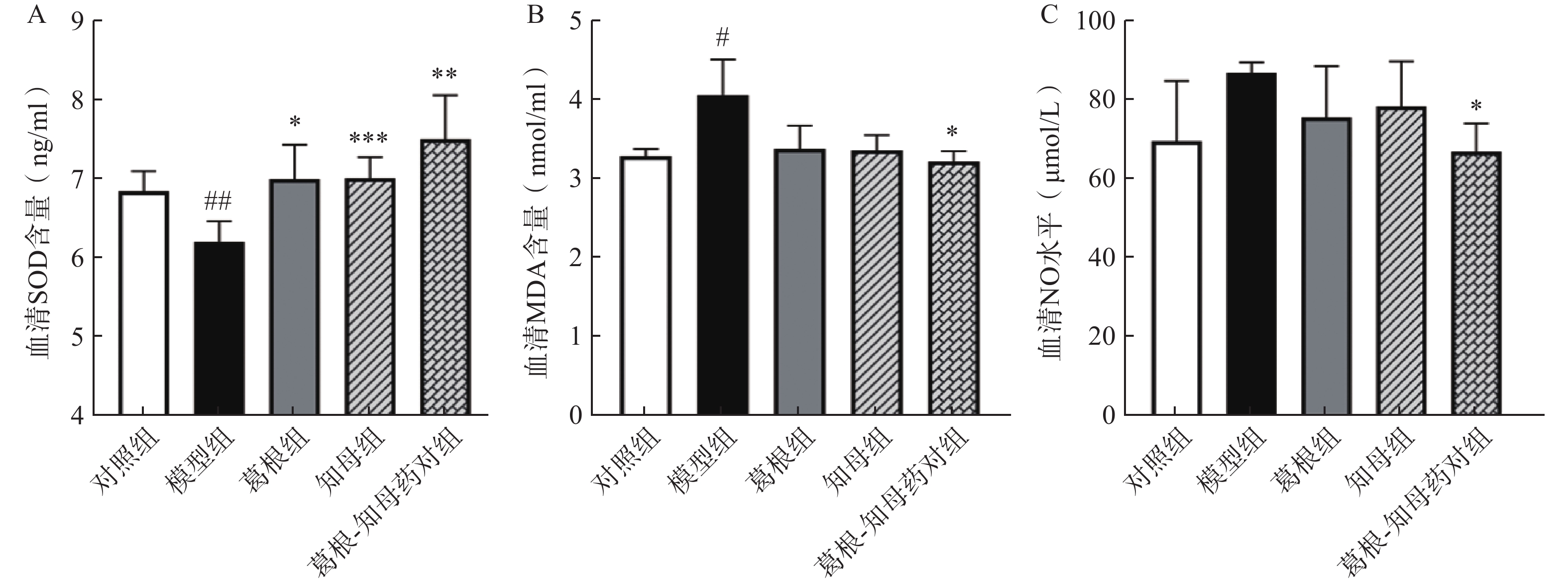
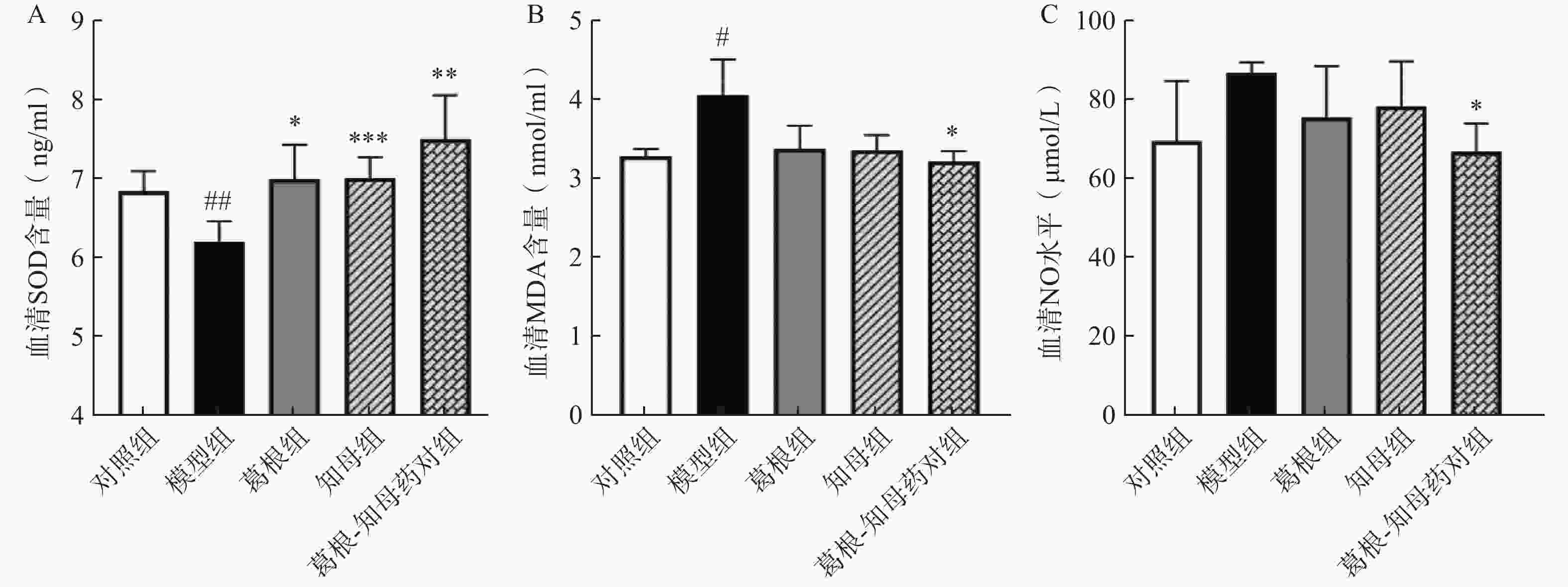
与对照组相比,模型组大鼠血清NO水平相对升高,MDA水平显著升高(P<0.05),SOD含量极显著降低(P<0.01)。中药干预后,各给药组血清NO和MDA水平出现不同程度降低,其中葛根-知母药对组降低效果最为明显,与模型组间差异具有统计学意义(P<0.05)。葛根、知母和葛根-知母药对给药组血清SOD含量较模型组均有所回调,组间差异具有统计学意义(P<0.05),见图2。
-
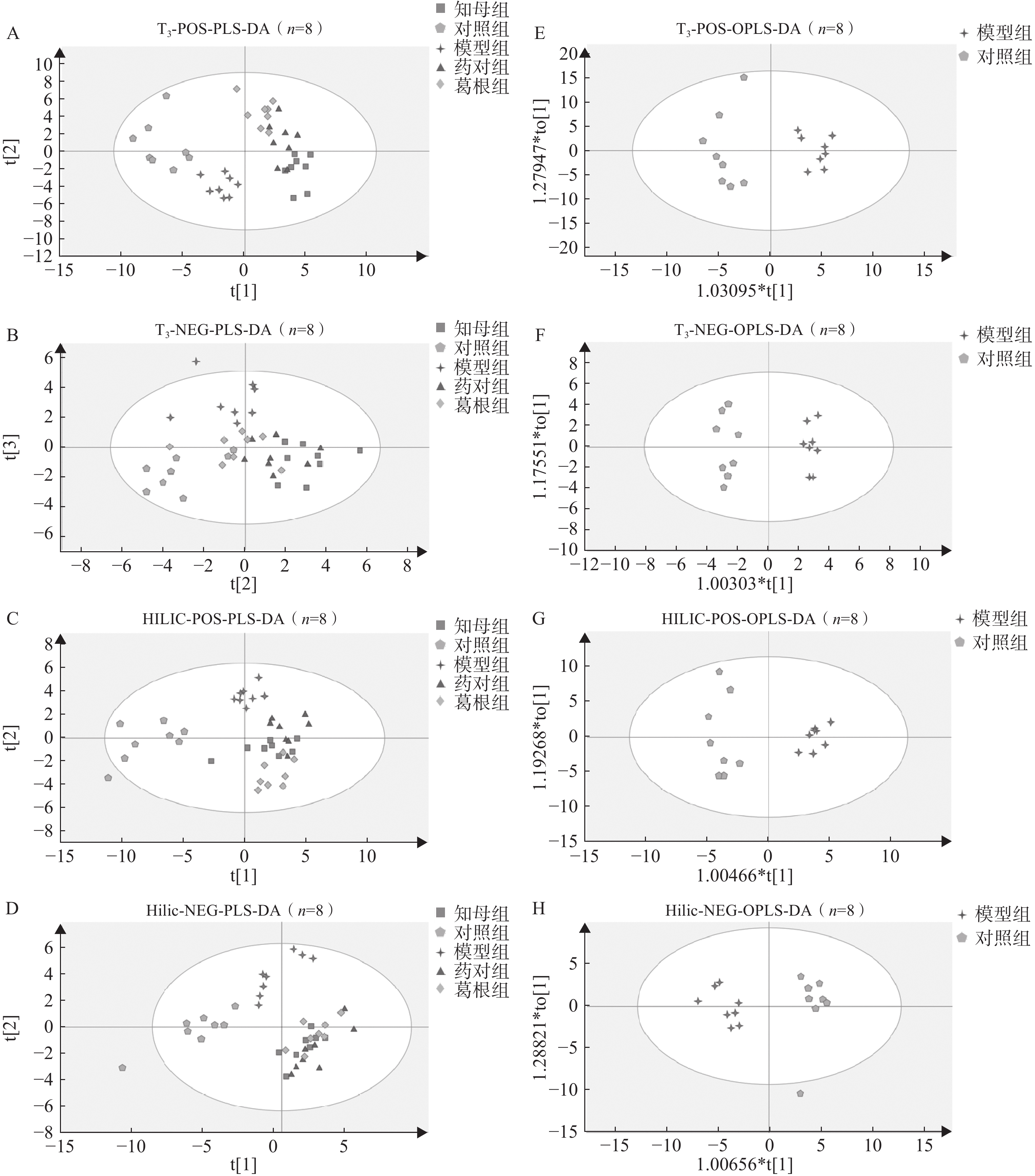
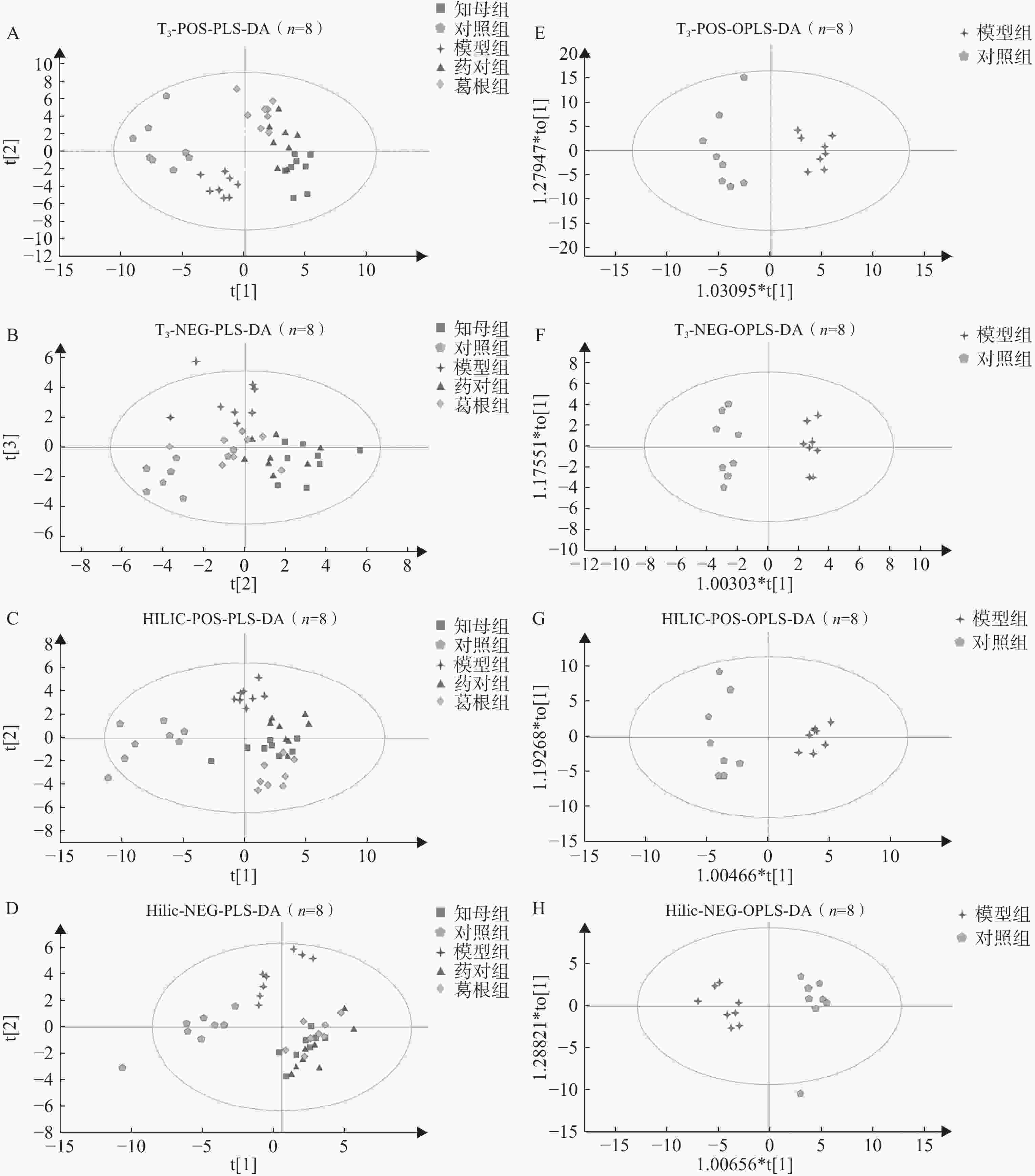
血清样本经UHPLC-Q/TOF-MS分析后得到各组大鼠血清代谢图谱,不同色谱柱分析条件下各组大鼠血清代谢轮廓存在一定差异。多元统计分析结果表明(图3),在PLS-DA多组分析模型中,空白对照组、AD模型组和3个中药干预组组间区分度较好,组内差异相对较小。PLS-DA模型200次置换检验结果显示,Q2回归线与Y轴截距小于0,R2和Q2曲线斜率始终为正值,且Q2<R2,表明模型未出现过拟合,具有相对可靠的解释和预测能力。在OPLS-DA模型中,不同分析条件下,AD模型组与空白对照组间完全分离,表明模型组与对照组间具有显著组间差异,CV-ANOVA验证结果证实所建立的OPLS-DA模型未出现过拟合,具备解释和预测能力。
-
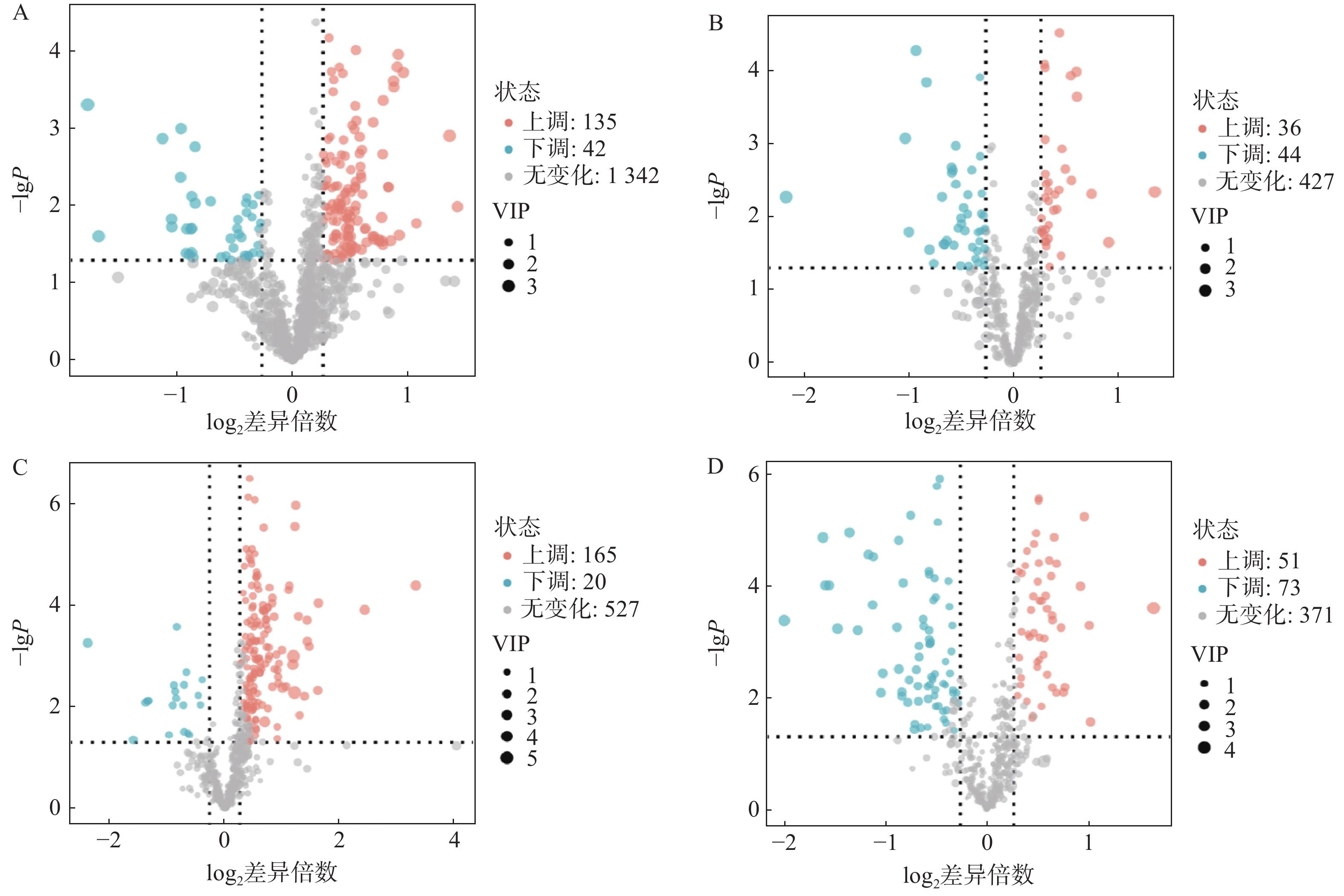
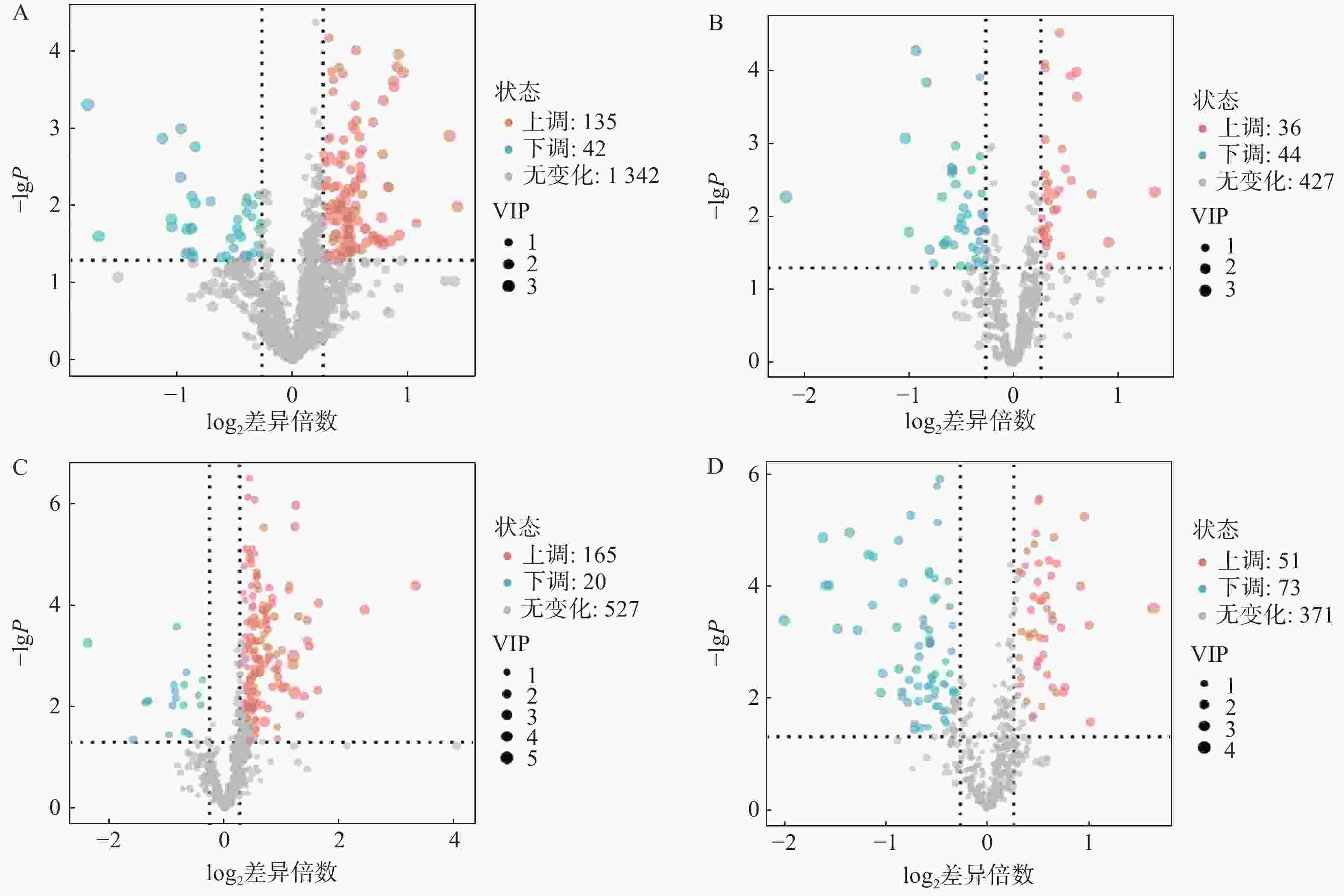
对T3柱和HILIC柱正、负离子模式下的代谢物信息进行差异化分析,以VIP值>1、P<0.05和FC>1.2或FC<0.8作为筛选标准,对不同模式下空白对照组与AD模型组的差异代谢物进行筛选,并以火山图形式呈现(图4)。图中橙色标记点为显著上调代谢物,蓝色标记点为显著下调代谢物。
利用HMDB数据库对差异代谢物质谱信息进行匹配和鉴定,在AD模型组与对照组间鉴定出70个AD相关的潜在生物标志物,其中由HILIC柱鉴定得到31个代谢物,T3柱鉴定得到45个代谢物,T3和HILIC柱共同鉴定得到的代谢物6个,具体如表1所示。
序号 代谢物 色谱柱 分子质量(m/z) 化学式 加合离子 趋势 相关通路 P 值 1 2-羟基丁酸 T3 127.0362 C4H8O3 M+Na ↑ 丙酸代谢 1.22E-03 2 肌酸 T3 132.0781 C4H9N3O2 M+H ↑# 甘氨酸、丝氨酸和苏氨酸代谢 6.98E-03 3 脯氨酸 T3、HILIC 138.0553 C5H9NO2 M+Na ↓#* 精氨酸和脯氨酸代谢 4.20E-03 4 L-天冬氨酸 T3 133.0606 C4H8N2O3 M+H ↑# 丙氨酸、天冬氨酸和
谷氨酸代谢2.36E-02 5 L-乙酰基肉碱 T3 204.1218 C9H17NO4 M+H ↑ 不饱和脂肪酸的生物合成 1.26E-03 6 棕榈酰肉碱 T3 400.3424 C23H45NO4 M+H ↑#* 脂肪酸降解 2.38E-04 7 喹啉酸 T3 168.0271 C7H5NO4 M+H ↑ 烟酸和烟酰胺代谢 4.97E-04 8 焦谷氨酸 T3 128.0329 C5H7NO3 M-H ↓# 谷胱甘肽代谢 2.89E-02 9 3b-羟基-5-胆酸 T3 357.2789 C24H38O3 M+H-H2O ↑ − 1.01E-02 10 香草酸 T3 151.0361 C8H8O4 M+H-H2O ↑ − 3.05E-03 11 肌酸酐 T3 136.0491 C4H7N3O M+Na ↑# − 1.89E-04 12 戊烯二酸 T3 153.0198 C5H6O4 M+Na ↑ − 8.96E-03 13 亚油酸 T3 303.2327 C18H32O2 M+Na ↑# 亚油酸代谢 2.45E-02 14 4-羟基丁酸 T3 103.0382 C4H8O3 M-H ↑# − 4.49E-03 15 糖原 T3 689.2111 C24H42O21 M+Na ↑ 淀粉和蔗糖代谢 2.24E-02 16 肉豆蔻酸 T3 211.2038 C14H28O2 M+H-H2O ↑# 脂肪酸生物合成 4.15E-02 17 丙酰肉碱 T3 218.1383 C10H19NO4 M+H ↓# 支链脂肪酸的氧化 1.97E-02 18 硬脂酰肉碱 T3 428.3734 C25H50NO4 M+H ↑#* 长链饱和脂肪酸的
线粒体β氧化2.84E-04 19 花生四烯酸 T3 327.232 C20H32O2 M+Na ↑#* 花生四烯酸代谢 1.53E-02 20 N1乙酰精胺 T3 267.208 C12H28N4O M+Na ↑# 赖氨酸降解 3.71E-02 21 N6, N6, N6-三甲基-L-赖氨酸 T3 189.16 C9H20N2O2 M+H ↑# α-亚麻酸代谢 4.44E-02 22 α-亚麻酸 T3 279.2316 C18H30O2 M+H ↑#* 初级胆汁酸生物合成 2.80E-02 23 24羟基胆固醇 T3 425.343 C27H46O2 M+Na ↑# 半胱氨酸和蛋氨酸代谢 8.17E-04 24 2-氧代-4-甲硫基丁酸 T3 131.0189 C5H8O3S M+H-H2O ↑ 不饱和脂肪酸的生物合成 1.14E-02 25 二十碳五烯酸 T3 285.2212 C20H30O2 M+H-H2O ↑# − 2.45E-02 26 油酰乙醇酰胺 T3 348.2891 C20H39NO2 M+Na ↑# − 8.42E-03 27 吲哚-3-丙酸 T3 190.0858 C11H11NO2 M+H ↓# − 4.79E-04 28 棕榈油酸 T3 237.2193 C16H30O2 M+H-H2O ↑#* − 5.79E-03 29 15(S)-羟基二十碳三烯酸 T3 345.2341 C20H34O3 M+Na ↑# − 9.83E-03 30 十四酰肉碱 T3、HILIC 372.3103 C21H41NO4 M+H ↑# − 1.15E-02 31 3-羟基马尿酸 T3 178.0501 C9H9NO4 M+H-H2O ↓#* − 9.53E-03 32 18-羟基花生四烯酸 T3 343.225 C20H32O3 M+Na ↑# − 3.91E-02 33 亚麻酰基肉碱 T3 424.3414 C25H46NO4 M+H ↑# − 4.25E-04 34 LysoPC(15:0/0:0) T3 526.3057 C23H48NO7P M+FA-H ↓#* − 2.55E-02 35 PC(18:1(9Z)e/2:0) T3 550.3872 C28H56NO7P M+H ↑#* − 2.11E-03 36 7-酮胆固醇 T3 401.3455 C27H44O2 M+H ↑#* − 1.08E-04 37 9-十六碳烯酰肉碱 T3 398.3152 C23H43NO4 M+H ↑# − 9.43E-05 38 16(17)-EpDPE T3 343.2219 C22H32O3 M-H ↑#* − 3.33E-02 39 十八烯酰肉碱 T3 426.3578 C25H47NO4 M+H ↑# − 1.84E-04 40 肉豆蔻酰肉碱 T3 370.2951 C21H39NO4 M+H ↑ − 4.25E-03 41 DL-乙酰肉碱 T3 204.1227 C9H17NO4 M+H ↑ 嘧啶代谢 1.85E-03 42 胞苷一磷酸 HILIC 368.0407 C9H14N3O8P M+FA-H ↓# 甘氨酸、丝氨酸和苏氨酸代谢 2.74E-02 43 胆碱 HILIC 86.0963 C5H14NO M+H-H2O ↑ 初级胆汁酸生物合成 1.20E-02 44 甘胆酸 HILIC 466.33 C26H43NO6 M+H ↑#* 苯丙氨酸、酪氨酸和色氨酸生物合成 2.43E-04 45 L-酪氨酸 HILIC 182.0812 C9H11NO3 M+H ↑#* 苯丙氨酸、酪氨酸和色氨酸生物合成 2.89E-03 46 苯丙氨酸 HILIC 166.0862 C9H11NO2 M+H ↑ 嘌呤代谢 1.68E-03 47 肌苷酸 HILIC 383.0262 C10H13N4O8P M+Cl ↓#* 丙氨酸、天冬氨酸和
谷氨酸代谢9.85E-04 48 L-天门冬氨酸 HILIC 134.0433 C4H7NO4 M+H ↑ 苯丙氨酸、酪氨酸和色氨酸生物合成 5.14E-04 49 苯丙酮酸 HILIC 165.0546 C9H8O3 M+H ↑#* 嘧啶代谢 6.25E-03 50 乳清酸 HILIC、T3 179.0029 C5H4N2O4 M+Na ↓#* 鞘脂代谢 3.04E-02 51 鞘氨醇 HILIC 302.3059 C18H39NO2 M+H ↑# 酪氨酸代谢 6.59E-04 52 香草扁桃酸 HILIC 233.0192 C9H10O5 M+Cl ↑#* 酪氨酸代谢 6.72E-05 53 酪胺 HILIC 120.079 C8H11NO M+H-H2O ↑ − 2.50E-03 54 3-氧代-4, 6 -胆二烯酸 HILIC 393.2315 C24H34O3 M+Na ↑# 初级胆汁酸生物合成 1.46E-02 55 鹅去氧胆酸 HILIC 437.2877 C24H40O4 M+FA-H ↑#* 丙氨酸、天冬氨酸和
谷氨酸代谢7.30E-04 56 谷氨酰胺 HILIC 169.0584 C5H10N2O3 M+Na ↑# − 5.06E-04 57 亮氨酸 HILIC 133.0855 C6H12O3 M+H ↑ − 5.18E-03 58 高-L-精氨酸 HILIC 189.1292 C7H16N4O2 M+H ↑#* − 1.22E-02 59 马尿酸 HILIC、T3 178.0516 C9H9NO3 M-H ↓# − 2.94E-02 60 牛磺胆酸3-硫酸盐 HILIC 596.2653 C26H45NO10S2 M+H ↑ − 1.78E-05 61 鹅去氧胆酸3-硫酸盐 HILIC 455.2515 C24H40O7S M+H-H2O ↑ 半胱氨酸和蛋氨酸代谢 2.76E-03 62 硫代半胱氨酸 HILIC 187.9645 C3H7NO2S2 M+Cl ↓# 亚油酸代谢 8.23E-05 63 13-L-过氧化氢亚油酸 HILIC 311.2187 C18H32O4 M-H ↓# − 4.63E-03 64 S-亚硝基谷胱甘肽 HILIC 381.0763 C10H16N4O7S M+FA-H ↓#* 鞘脂代谢 8.86E-05 65 LacCer(d18:1/12:0) HILIC 806.5705 C42H79NO13 M+H ↑ 花生四烯酸代谢 1.30E-04 66 LysoPC(14:0/0:0) HILIC、T3 512.3009 C22H46NO7P M+FA-H ↓ − 3.34E-02 67 2-(14,15-环氧二十碳三烯酰基)甘油 HILIC 395.2749 C23H38O5 M+H ↑ − 1.41E-03 68 赖氨酰苯丙氨酸 HILIC 294.1891 C15H23N3O3 M+H ↑ 醚脂代谢 2.52E-04 69 二十四碳四烯酸肉碱 HILIC、T3 526.3786 C31H53NO4 M+Na ↑ − 2.98E-05 70 1-(11Z二十二碳烯酰基)-3-磷酸甘油酯 HILIC 515.3163 C25H49O7P M+Na ↑ − 8.91E-07 注:↑表示模型组较对照组相对升高趋势,↓表示模型组较对照组相对下降趋势,P值为模型组与对照组间代谢物水平的t检验计算结果;
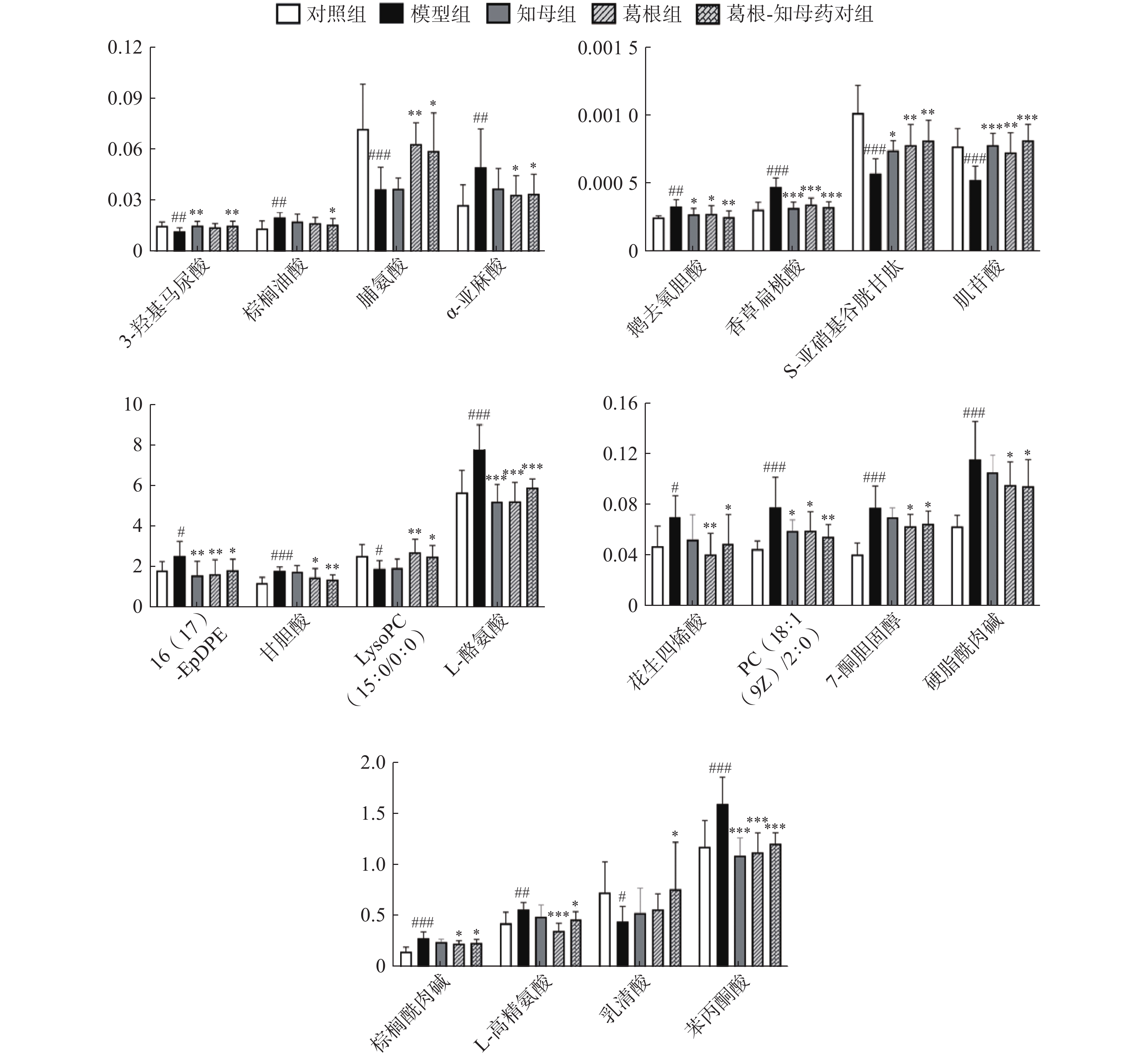
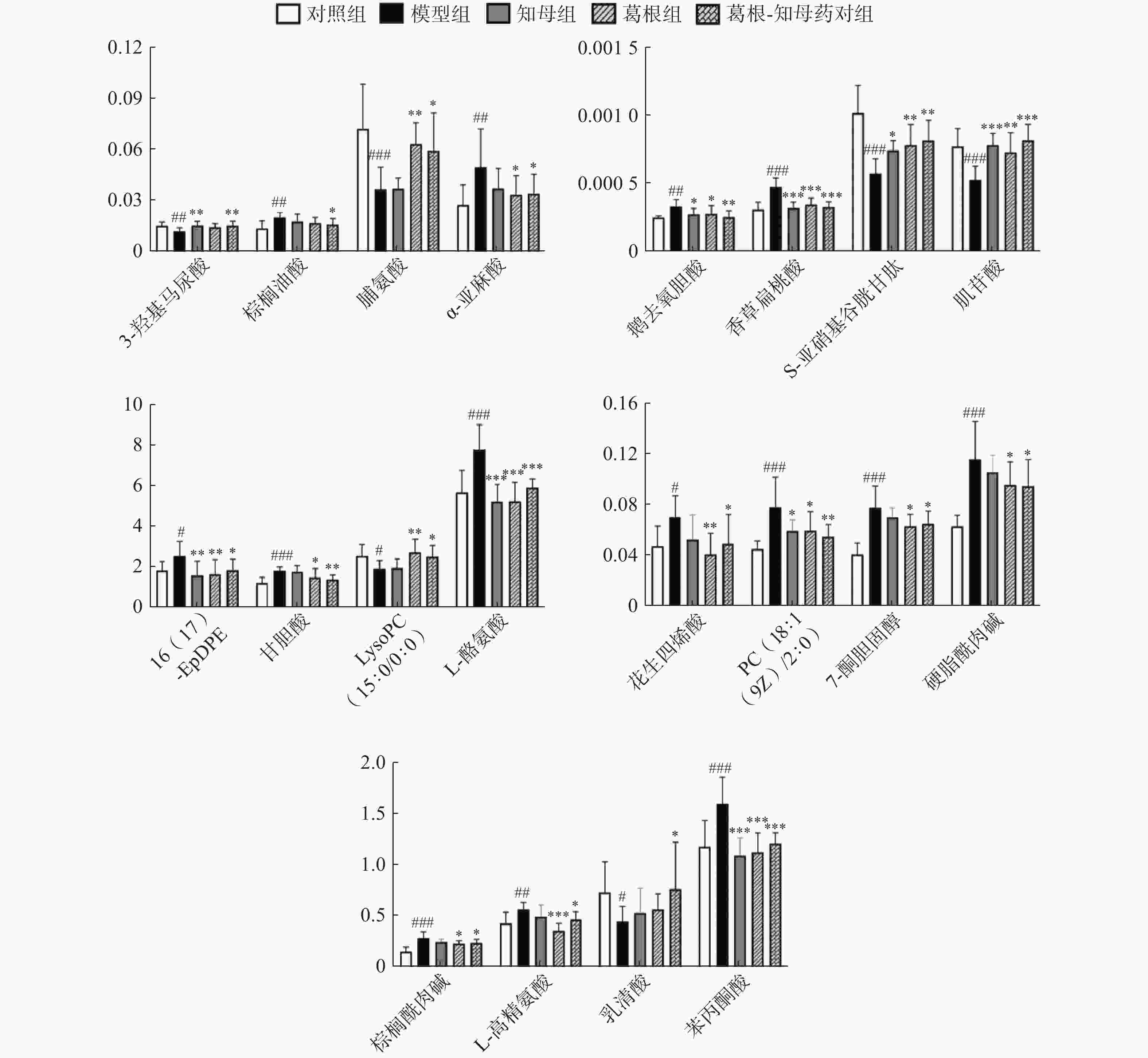
#表示代谢物经葛根-知母药对干预后具有回调趋势(共47个),*表示代谢物(共20个)经葛根-知母药对干预后回调差异具有统计学意义(P<0.05)。 -
利用各组间的FC值变化情况判断药对干预后的回调代谢物。对于具有回调趋势的代谢物多组间变化情况进行单因素方差分析,P<0.05的代谢物确定为药对干预后显著回调的差异代谢物。结果显示,葛根-知母药对干预后出现回调的差异代谢物共计47个,其中,显著回调代谢物20个(表1和图5)。
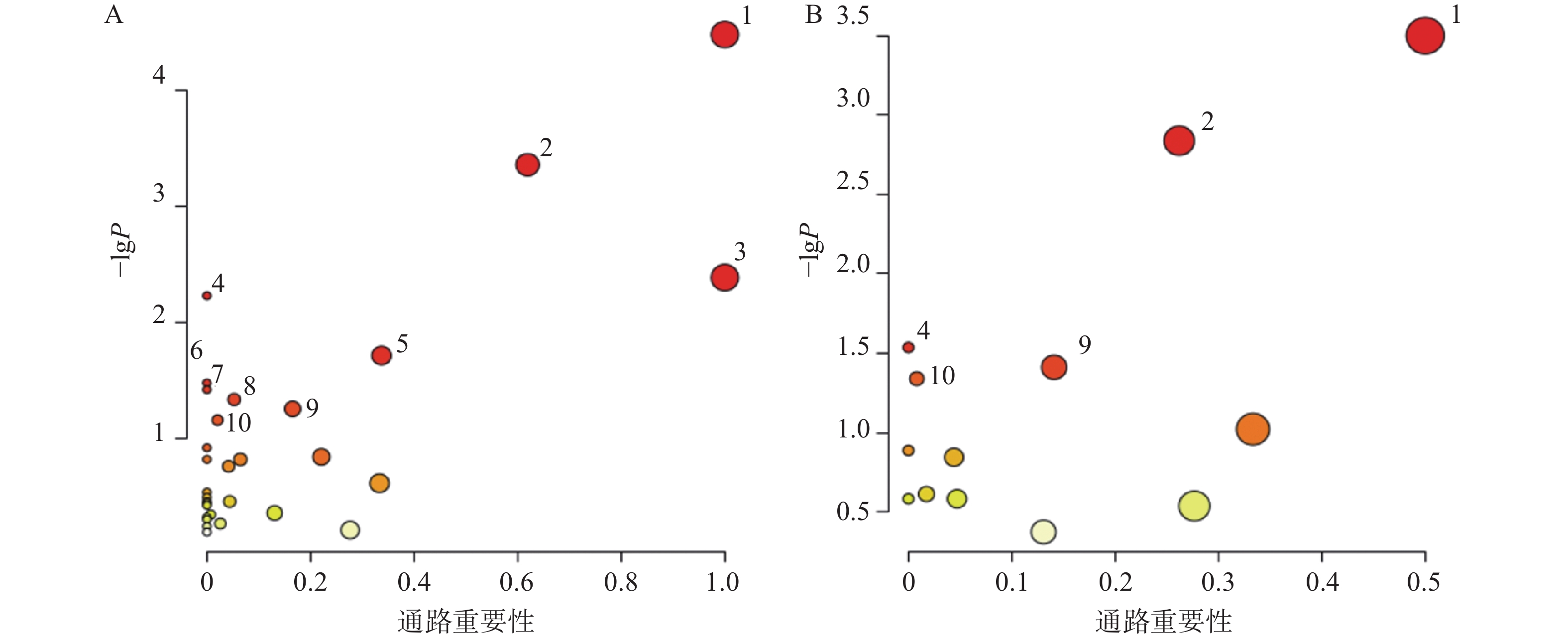
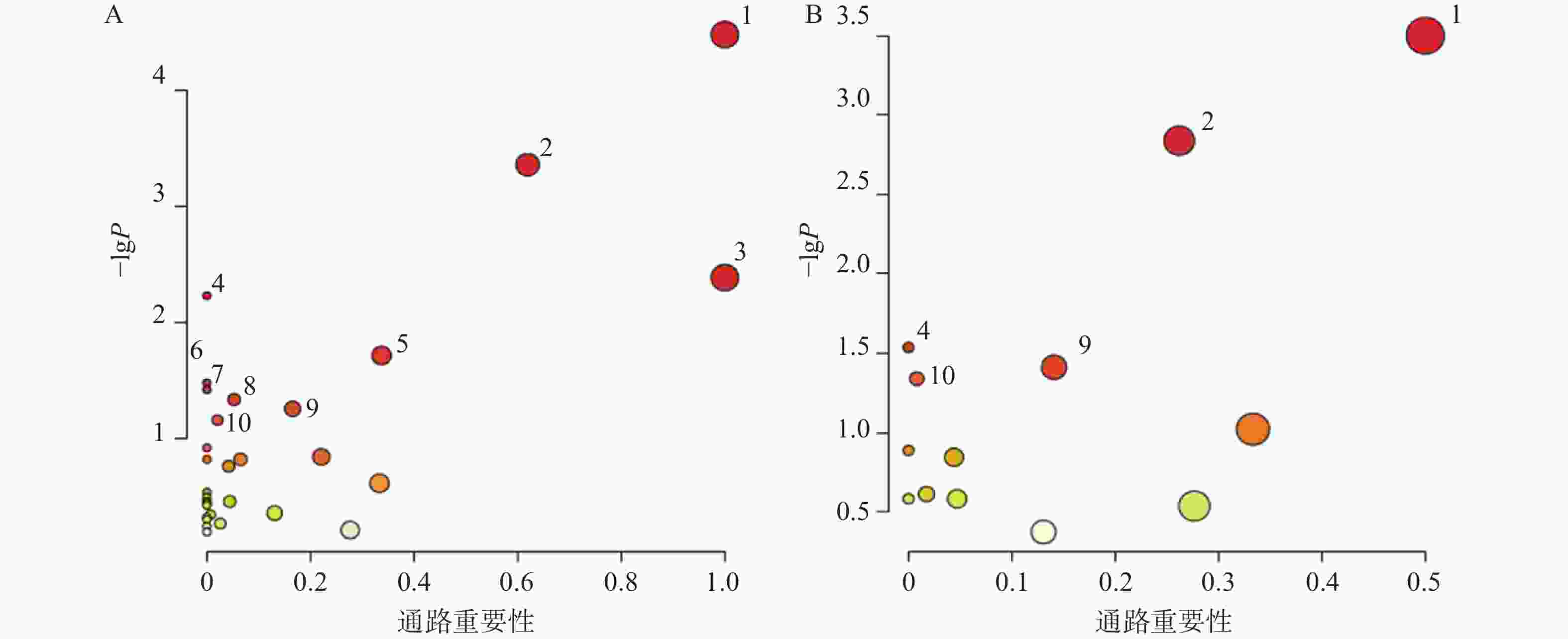
对70个AD相关的差异代谢物和葛根-知母药对干预后显著回调的20个差异代谢物分别进行通路富集分析后发现(图6),AD模型大鼠潜在疾病生物标志物涉及通路主要包括苯丙氨酸、酪氨酸和色氨酸生物合成、苯丙氨酸代谢、亚油酸代谢、不饱和脂肪酸的生物合成、丙氨酸、天冬氨酸和谷氨酸代谢、精氨酸生物合成、酪氨酸代谢、嘧啶代谢等。葛根-知母药对干预可对苯丙氨酸、酪氨酸和色氨酸生物合成、苯丙氨酸代谢、不饱和脂肪酸的生物合成、酪氨酸代谢和初级胆汁酸生物合成等通路产生回调影响。
-
AD是一种复杂的中枢神经系统异质性疾病,具体病因尚不明确。目前已知可导致AD的因素包括基因突变、氧化应激、神经炎症以及多种环境和疾病因素[11] 。衰老被认为是AD最相关的危险因素[12] ,啮齿类动物长期摄入D-gal可产生包括氧化应激、炎症反应在内的多种与人类相似的衰老相关变化[13] 。铝元素可通过促进中枢神经系统炎症反应、降低脑中SOD活性,影响胆碱能神经传递、促进Tau蛋白磷酸化等形式诱导神经毒性,过量铝暴露与AD等中枢神经系统退行性疾病进展相关[14] 。研究表明,D-gal与AlCl3联合应用可产生类似于自然衰老的变化及AD相关特征[15] 。因此,本研究选取D-gal与AlCl3联合造模,通过大鼠长程给药模拟和还原AD相关的病程和病理变化,力求更加精准地反映AD患者体内代谢分子水平变化。药效学实验表明,D-gal和AlCl3联合给药后大鼠学习和记忆能力明显下降,体内氧化应激和炎症相关因子水平发生变化,表明该模型成功模拟AD相关的病理变化和特征。而葛根-知母药对干预可显著改善AD模型大鼠的学习和记忆能力,并回调SOD、NO和MDA等相关生化指标,改善和回调效果优于单药,表明葛根和知母配伍后在AD防治中具有一定的增效作用,具备进一步研究的价值。
代谢组学结果表明,葛根-知母药对可以显著回调血清中20种代谢物,主要涉及苯丙氨酸、酪氨酸和色氨酸生物合成、苯丙氨酸代谢、不饱和脂肪酸的生物合成、酪氨酸代谢等途径。酪氨酸是一些神经递质或者神经调节剂的前体。Liu[16]等对尸源样本进行非靶向和靶向代谢组学分析发现,AD患者海马中苯丙酮酸含量普遍上调,表明苯丙氨酸代谢失调可能是AD病理形成的重要机制。本研究同样发现AD模型大鼠血清中苯丙酮酸水平较对照组显著升高,葛根-知母药对可以显著回调苯丙酮酸至生理水平。脯氨酸是一种非必需氨基酸,参与氧化还原调控和细胞凋亡,既是活性氧(ROS)清除剂,也是ROS生产者,因此,平衡脯氨酸水平和脯氨酸相关代谢酶活性对维系细胞生理功能至关重要。脯氨酸代谢可能会通过ROS、细胞衰老和细胞免疫等机制影响神经元功能[17] 。Xu等[18]整合代谢组学与蛋白质组学结果发现,脱脂核桃粉可以通过升高小鼠脑组织中脯氨酸等多种氨基酸水平发挥对东莨菪碱诱导的AD小鼠神经保护机制。这与本实验结果相似,葛根-知母药对可以通过回调脯氨酸水平发挥AD防治作用。
此外,本研究发现多种回调代谢物与脂质代谢密切相关。花生四烯酸和α-亚麻酸是由多不饱和脂肪酸氧化产生的脂氧化物,广泛参与机体炎症、免疫等多种生理病理进程。花生四烯酸升高可进一步提高氧化应激水平,与AD等疾病进程紧密相关[19] 。AD与胆汁酸代谢异常之间存在紧密关联,这可能与肠-肝-脑轴机制相关。有研究表明AD患者血浆中胆汁酸水平升高[20] 。磷脂是保持细胞膜完整性的主要物质,溶血磷脂酰胆碱是磷脂的降解产物,与磷脂代谢密切相关,磷脂代谢异常可导致溶血磷脂酰胆碱下调,表现为细胞凋亡及信号转导异常,是AD的潜在诱因之一[21] 。本研究表明,葛根-知母药对的AD防治作用效果可能与显著回调血清中花生四烯酸、α-亚麻酸、甘胆酸、鹅去氧胆酸和溶血磷脂酰胆碱水平相关。
ROS产生与消除之间的不平衡被称为氧化应激,氧化应激是AD等疾病的关键因素和共同点。NO是ROS的一种,也是神经传递和炎症相关的重要因素。高水平ROS会触发不饱和脂肪酸的脂质过氧化,导致MDA等高反应性化合物的产生,因此MDA是脂质过氧化和氧化应激的标志。SOD是抵消ROS有害影响最有效的一线防御机制。SOD可以通过去除超氧自由基防止更具破坏力的过氧亚硝酸盐生成,并维持体内NO在生理相关水平[22, 23] 。代谢组学结果提示,葛根-知母药对回调干预的多种途径与氧化应激和脂质过氧化相关。ELISA实验结果进一步表明,葛根-知母药对干预可提高AD大鼠体内SOD水平,回调NO和MDA至生理水平,提示葛根-知母药对可以通过调节氧化应激和脂质过氧化,维持体内NO的生理水平对AD产生防治作用。
综上,本文通过建立AD大鼠模型考察了葛根-知母药对防治AD的作用效果,药对药效优于单药;运用代谢组学策略揭示其改善AD大鼠学习和记忆能力相关的潜在代谢物和代谢路径,其作用机制可能与调节苯丙氨酸、酪氨酸和色氨酸生物合成等代谢通路、改善氧化应激和脂质过氧化水平等相关,为中药药对防治AD的临床应用和进一步开发提供了科学依据。
Study on the pharmacological effects and mechanism of Gegen-Zhimu herb pair in preventing and treating Alzheimer's disease by UHPLC-Q/TOF-MS metabolomics strategy
doi: 10.12206/j.issn.2097-2024.202409035
- Received Date: 2024-09-13
- Rev Recd Date: 2024-11-06
- Available Online: 2025-01-16
- Publish Date: 2025-01-25
-
Key words:
- Alzheimer's disease /
- metabolomics /
- Gegen-Zhimu /
- herb pair /
- mechanism
Abstract:
| Citation: | CHAO Liang, WANG Hui, SHEN Shuqi, YOU Piaoxue, JI Kaihong, HONG Zhanying. Study on the pharmacological effects and mechanism of Gegen-Zhimu herb pair in preventing and treating Alzheimer's disease by UHPLC-Q/TOF-MS metabolomics strategy[J]. Journal of Pharmaceutical Practice and Service, 2025, 43(1): 30-40. doi: 10.12206/j.issn.2097-2024.202409035 |







 DownLoad:
DownLoad: