-
社区获得性肺炎(CAP)是指在院外由于多种微生物引起的肺实质的急性感染,临床表现以发热、咳嗽、咯痰、气短、胸闷或胸痛等症状为主[1-2]。2019年全球疾病负担研究(GBD)的数据显示,包括肺炎和细支气管炎在内的下呼吸道感染影响了全球4.89亿人[3]。全球估计每年约有320万人死于CAP,超过了包括结核病、艾滋病毒感染和疟疾在内的所有其他感染,使其成为全球传染病死亡的主要原因,其中小于5岁的儿童及大于70岁的成年人是受影响最大的人群[4]。
近年来,有大量的临床研究、实验观察以及医学报道表明,中西医联合治疗CAP取得了较好的疗效。在使用抗菌药物的基础上,加用中药能够缩短病程,尽量减少细菌耐药性的发生。同时中药在治疗过程中有着多靶点、多环节的作用机制。金芪清疏颗粒(备案号:沪药制备字Z20200011001)是上海中医药大学附属岳阳中西医结合医院呼吸科专家在长期临床治疗肺脾气虚证呼吸道疾病基础上所得出的经验效方,以玉屏风散和四君子汤经典名方为基础加减化裁而成。它由金银花、黄芪、黄芩、柴胡、太子参、茯苓、白术、佩兰、防风和甘草10味药组成,具有益气清疏,健脾补肺固表,培土生金之效。现代药理学研究表明[5-15],方中所用诸药具有广泛抗炎、调节氧化应激、解热、抗菌、调节免疫等多种功效。但由于中药成分治疗靶点的复杂性,目前有关金芪清疏颗粒治疗CAP的具体机制尚不明确。本研究基于网络药理学、分子对接等方法探究其潜在作用机制,拟为后续实验研究提供理论基础。
-
本研究利用TCMSP数据库[16],以金银花、黄芪、黄芩、柴胡、太子参、茯苓、白术、佩兰、防风、甘草为关键词检索药物成分,设置口服生物利用度(OB)≥30%、类药性(DL)≥0.18,其他参数为默认值,进行药物成分筛选,通过查阅文献对不满足筛选要求,但含量高或者相关药物活性强的予以手动筛选添加。最终获取金芪清疏颗粒的活性成分,并通过PubChem数据库[17]获取活性成分SMILE号。若PubChem数据库未能检索出的活性成分,则通过TCMSP平台下载相关的活性成分的mol2文件,在novopro平台上将mol2文件转换为SMILE号。将所有活性成分的SMILE号导入SwissTargetPrediction数据库中获取各个化学成分的预测作用靶点,其中物种参数设置为Homo sapiens,置信度阈值(Probability>0)。利用Cytoscape3.10.0软件[18]构建金芪清疏颗粒活性成分与作用靶点网络,预测金芪清疏颗粒核心成分。
-
以“Community acquired pneumonia”为检索词,分别在OMIM、TTD[19]、DisGeNET和GeneCards 4个数据库中检索CAP作用靶点,种属选择“Homo sapiens”,重复靶点仅保留1个。
-
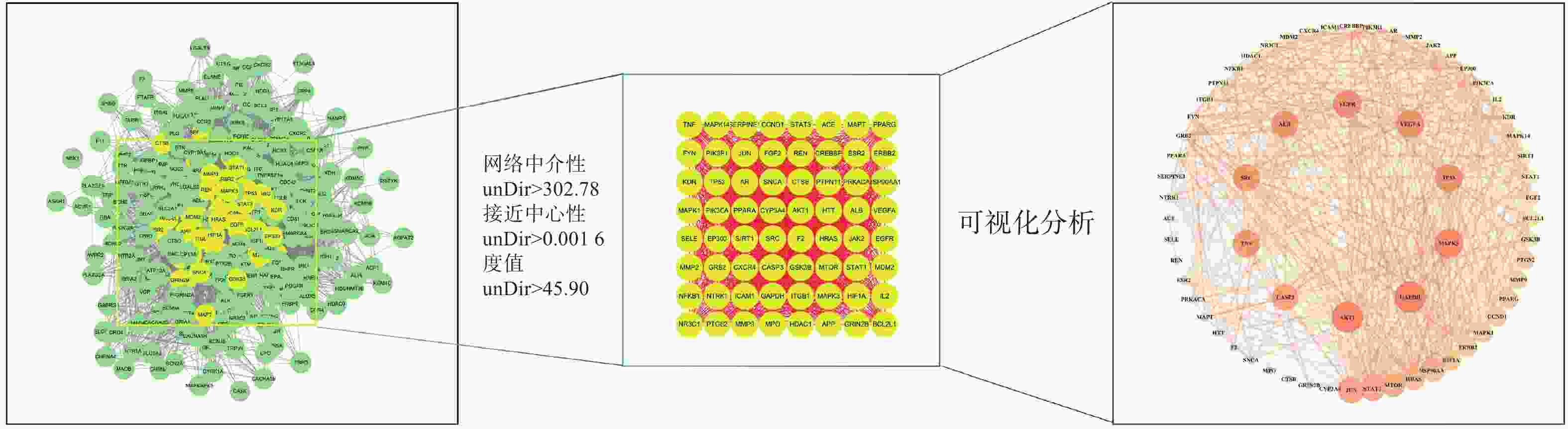
通过JVEEN(www.bioinformatics.com.cn) [20]将金芪清疏颗粒活性成分的预测靶点与CAP相关靶点进行映射,获得金芪清疏颗粒作用于CAP的潜在靶点。利用String平台构建金芪清疏颗粒作用于CAP的靶点蛋白相互作用(PPI)网络。再利用Cytoscape3.10.0软件的“Centiscape2.2”插件对PPI网络进行拓扑分析,以度数(Degree)、中介性(Betweenness)、接近中心性(Closeness)筛选出该网络的关键靶点,按Degree值进行排序,得出TOP9靶点为核心靶点,并通过Cytoscape3.10.0进行可视化分析。
-
将“1.3”项中得到的金芪清疏颗粒-CAP的关键靶点的编码基因导入DAVID 数据库,DAVID 数据库的基因列表中,选择 “OFFICIAL GENE SYM-BOL”, 选定物种为“Homo sapiens”,进行GO功能及KEGG通路富集分析,利用JVEEN制作富集气泡图,进行数据可视化。
-
从 TCMSP数据库查询“1.1”项中关键成分结构,并保存为 mol2 格式。从 PDB 数据库下载“1.3”项核心靶点的 3D 结构,保存为 PDB 格式,使用 PyMol软件删除蛋白结构的水分子和小分子配体,并导入 AutoDockTools 进行加氢等预处理。将活性成分和靶点蛋白均转换成 pdbqt 格式文件。最后运行 AutoDockTools 对活性成分和靶点蛋白分别进行对接,保存最低结合能数据作为分子对接的结果。
-
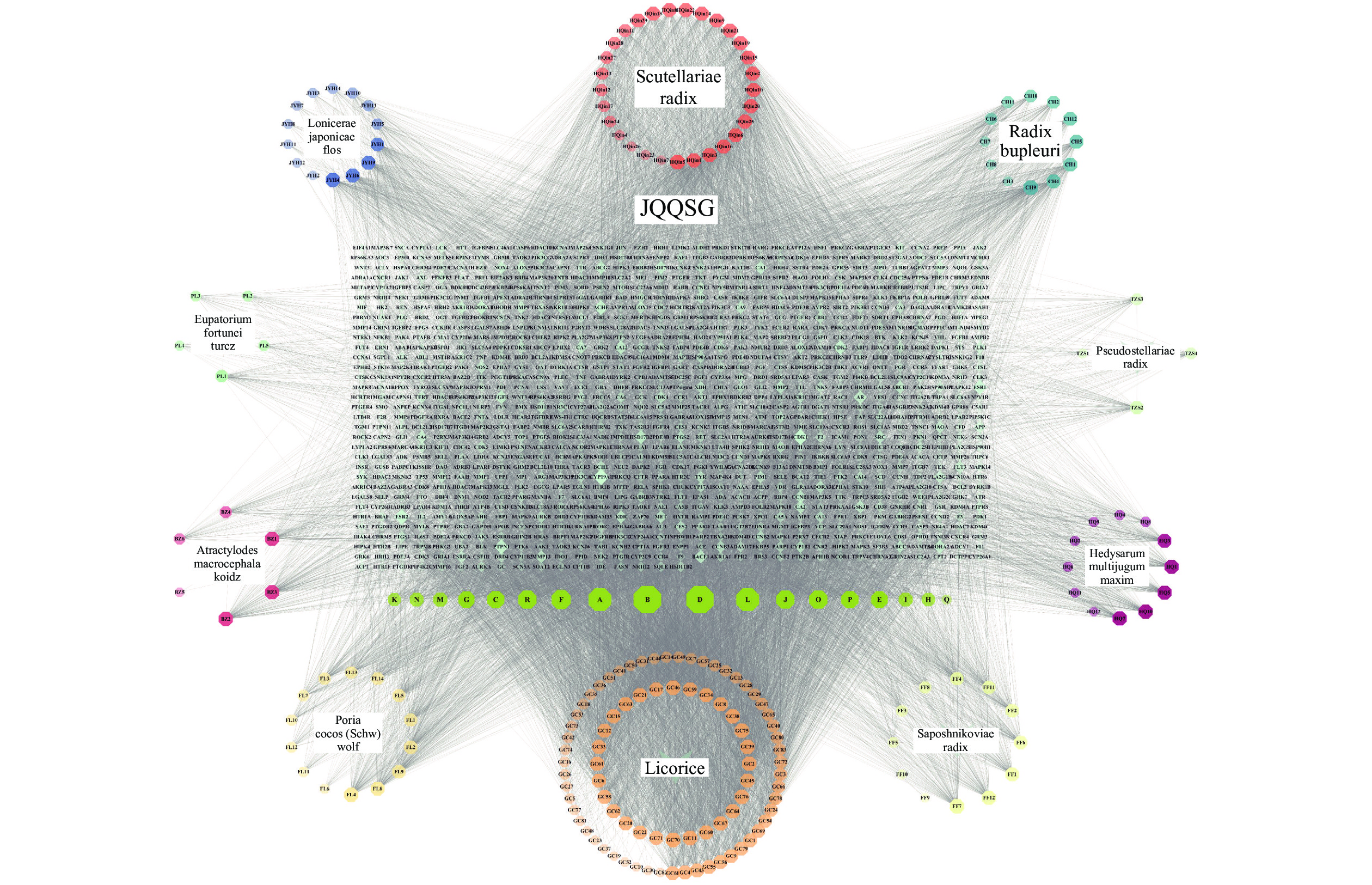
根据筛选得到金银花23个、黄芪21个、黄芩36个、柴胡17个、太子参8个、茯苓15个、白术7个、佩兰11个、防风18个和甘草92个活性成分,删去重复值及没有作用靶点的成分,共得到金芪清疏颗粒活性成分209个,通过Swiss Target Predic-tion数据库预测相应作用靶点,删去重复值共得到
1041 个作用靶点。通过Cytoscape3.10.0软件将活性成分-靶点进行可视化,得到“活性成分-靶点”相互作用网络。该网络共有1291 个节点和16408 条边,见图1。通过软件分析得出Degree值,Degree值越高,作用关系越密切,重要性越大,按Degree值排序,得出top 9的成分为:Quercetin、Kaempferol、Luteolin、Isorhamnetin、Mandenol、Jaranol、Wogonin、Acacetin和Sitosterol,见表1。化合物 分子结构 度值 中介中心性 接近中心性 口服生物利用度(%) 类药性 中药名称
成分代号(ID)Quercetin(槲皮素) 
420 0.00350184 0.37422037 46.43 0.28 金银花、黄芪、
太子参、甘草B Kaempferol(山柰酚) 
420 0.00343999 0.37422037 41.88 0.24 金银花、黄芪、
柴胡、甘草D Luteolin(木犀草素) 
315 0.00386583 0.37377633 31.16 0.25 金银花、太子参、佩兰 A Isorhamnetin
(异鼠李素)
315 0.0031137 0.37377633 49.60 0.31 黄芪、柴胡、
甘草L Mandenol
(亚麻油酸乙酯)
230 0.02557745 0.37223043 42.00 0.19 金银花、防风 F Jaranol(华良姜素) 
210 0.00361453 0.3735547 50.83 0.29 黄芪、甘草 J Wogonin(汉黄芩素) 
210 0.00350745 0.37399822 30.68 0.23 黄芩、防风 O Acacetin(金合欢素) 
210 0.00355992 0.37311223 34.97 0.24 黄芩、太子参 R Sitosterol(谷甾醇) 
180 0.00218402 0.35502959 36.91 0.75 黄芩、防风、
佩兰、甘草P -
以“Community acquired pneumonia”为检索词分别从OMIM、DisGeNET、GeneCards(评分>3.6)、TTD 4个数据库得到645、73、
1566和 3个疾病靶点,删去重复值后共得到2149 个CAP相关靶点。 -
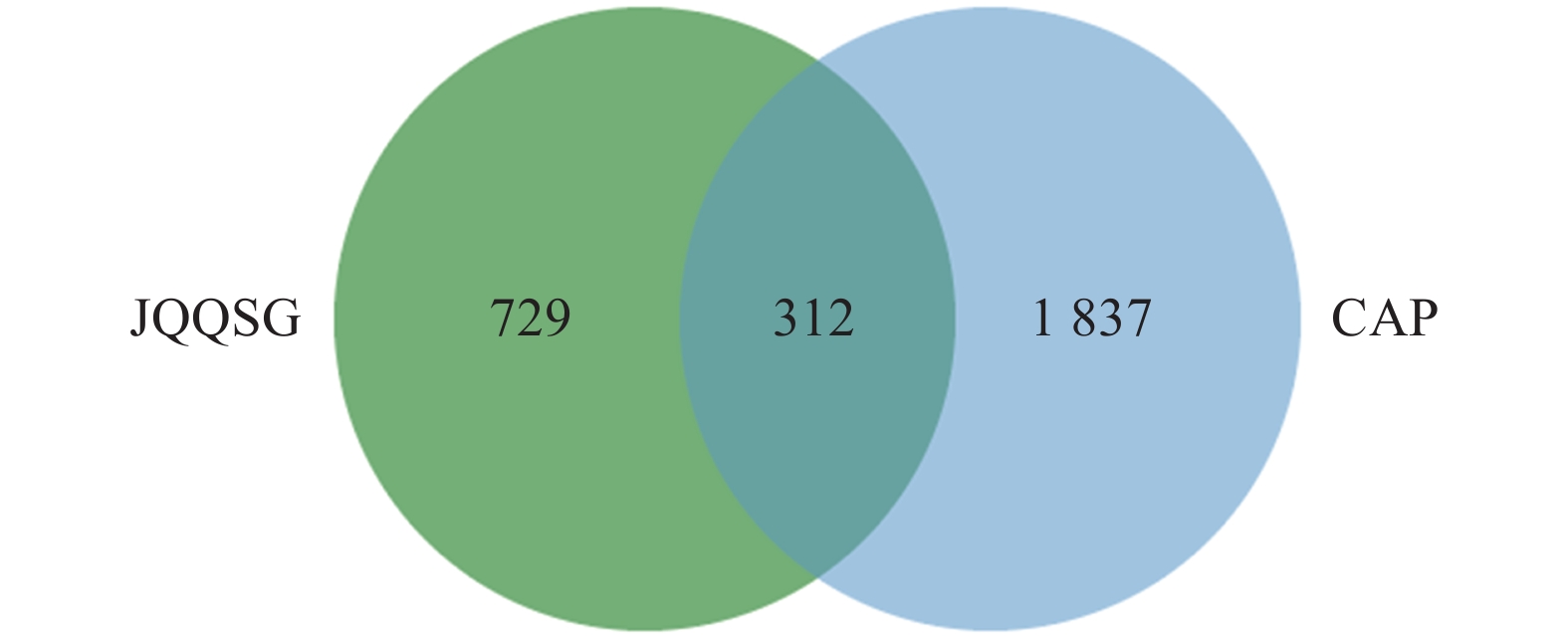
将
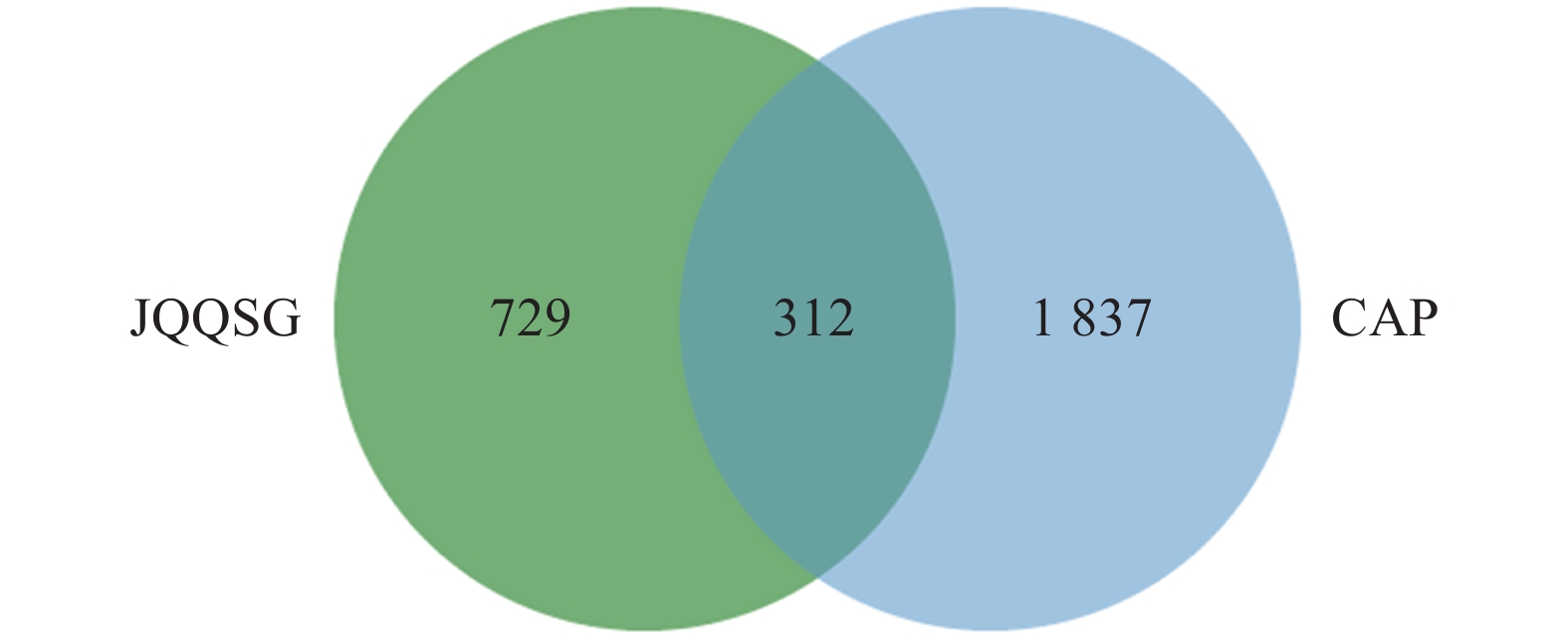
1041 个金芪清疏颗粒活性成分作用靶点与2149 个CAP相关靶点进行映射,得到312个共同靶点,绘制VEEN图,见图2。这些靶点被认为是金芪清疏颗粒治疗CAP的潜在靶点。 -
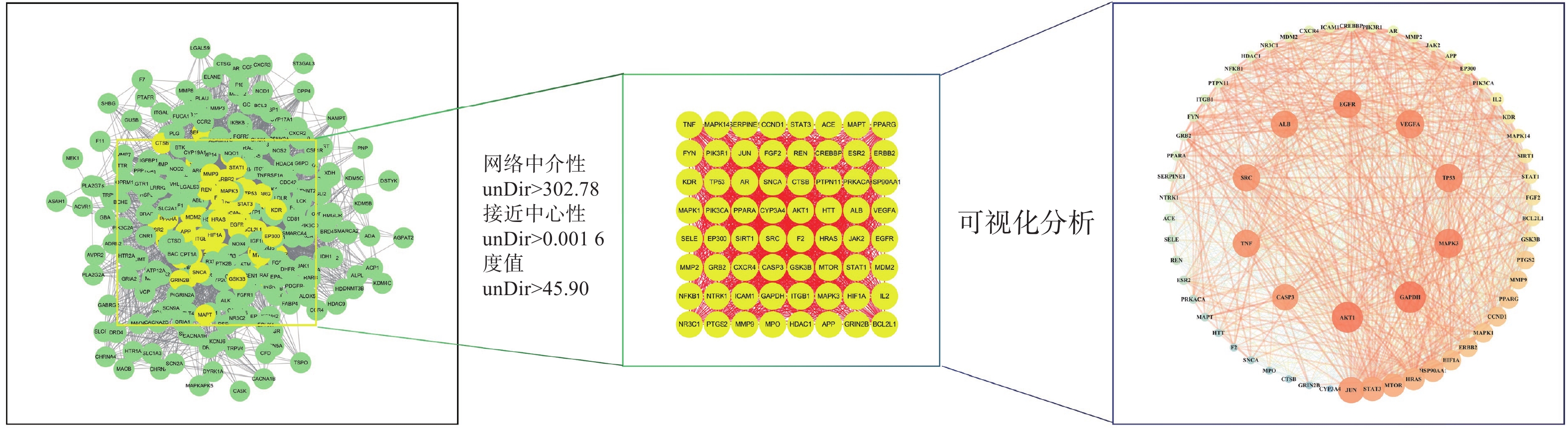
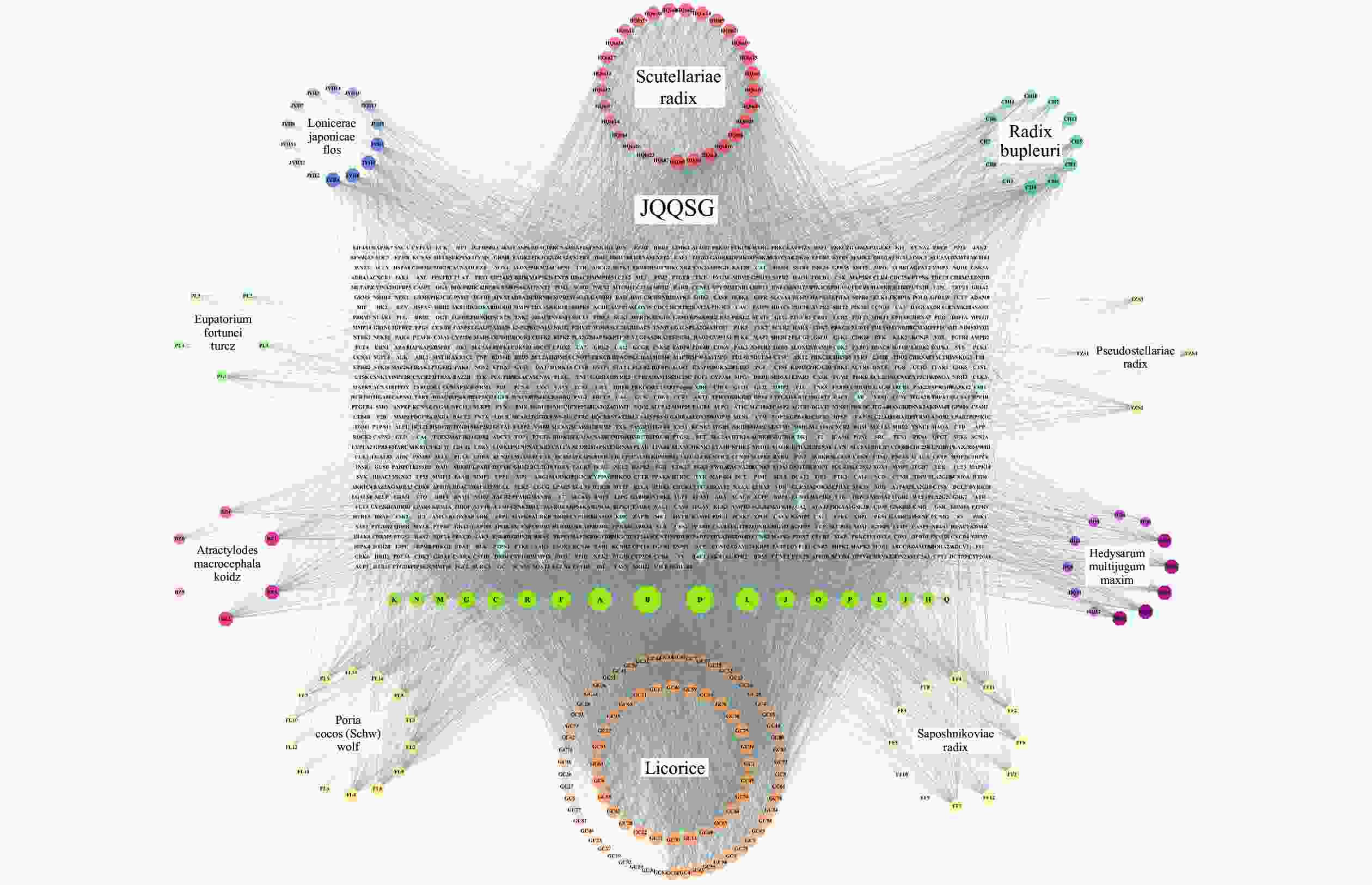
将312个作用靶点导入STRING,构建PPI网络。利用Centiscape2.2插件分析,得到该网络中介性unDir:302.778,接近中心性unDir:0.002,度值unDir:45.910,取大于这三个条件筛选出该网络关键靶点64个,利用Cytoscape3.10.0进行可视化分析(图3),结果得到TOP10靶点为:GAPDH、AKT1、MAPK3、TP53、EGFR、VEGFA、ALB、TNF、SRC和JUN,见表2。
名称 度值 中介中心性 接近中心性 GAPDH 61 0.0185 0.9692 AKT1 61 0.0195 0.9692 MAPK3 59 0.0167 0.9402 TP53 58 0.0146 0.9265 EGFR 58 0.0149 0.9265 VEGFA 58 0.0145 0.9265 ALB 57 0.0168 0.9130 TNF 57 0.0133 0.9130 SRC 57 0.0127 0.9130 JUN 56 0.0101 0.9000 -
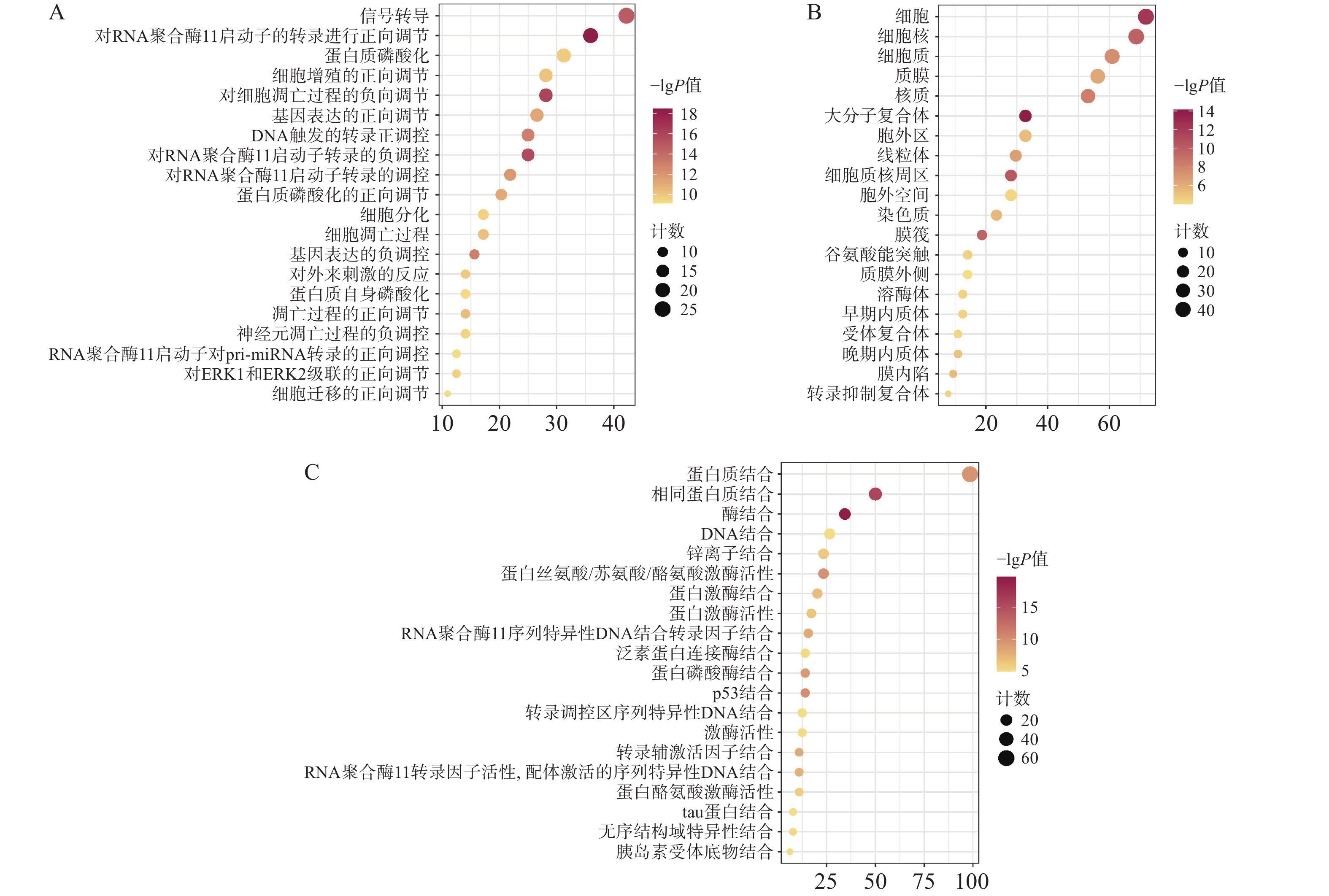
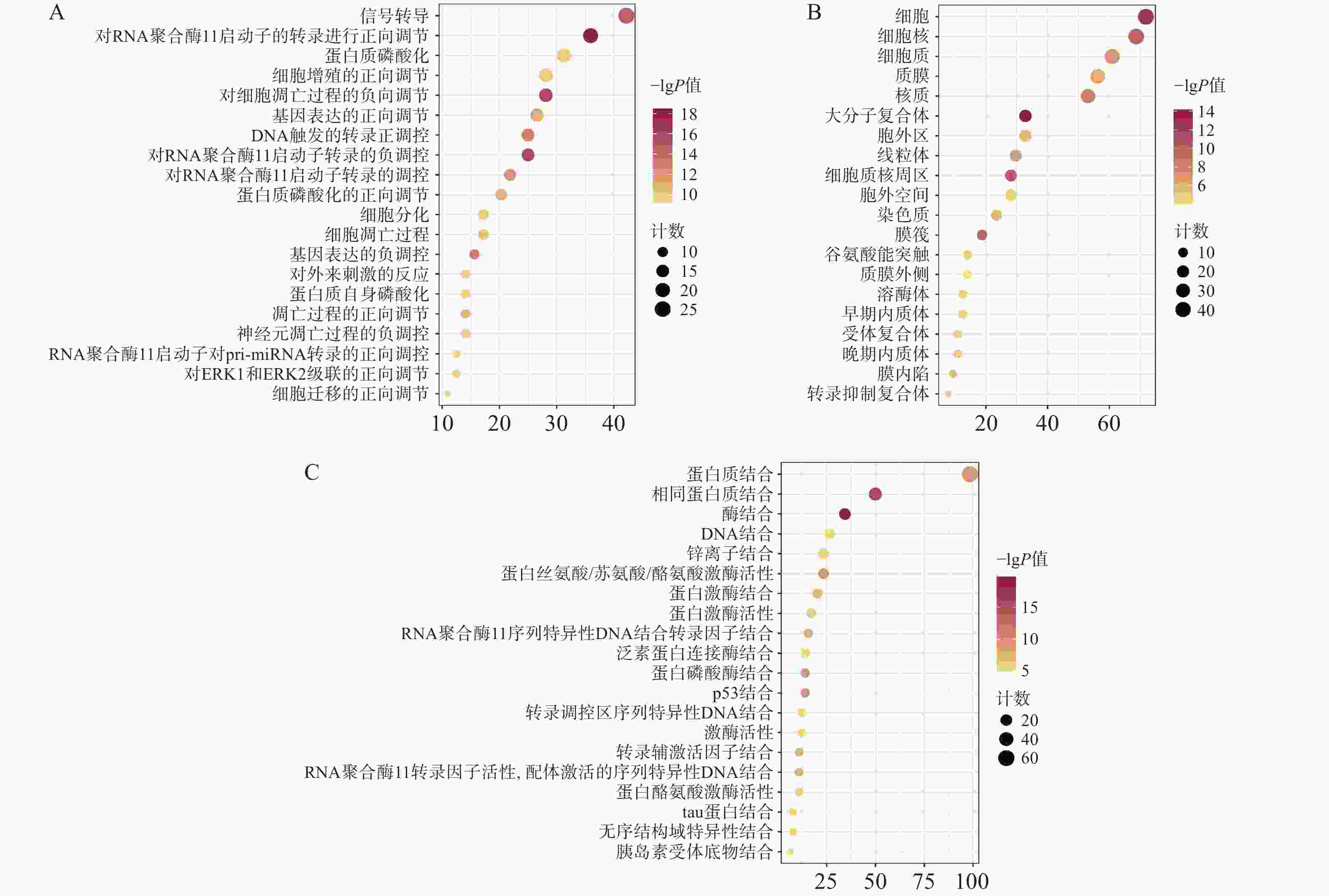
通过David数据库对64个核心靶点进行GO富集分析及KEGG分析。用Bioinformatics[21]制作富集气泡图。结果显示,共有571个生物过程、68个细胞组分、199个分子功能显示富集。据P<0.01进行筛选后排序,选择前20的结果。GO富集分析主要涉及在正向调节RNA聚合酶Ⅱ启动子转录,凋亡过程的负调控、信号转导、蛋白磷酸化等生物过程,见图4A;细胞质、细胞核、大分子复合物等细胞组分,见图4B;蛋白质结合、酶结合、蛋白质丝氨酸/苏氨酸/酪氨酸激酶活性,转录因子结合等分子功能,见图4C。
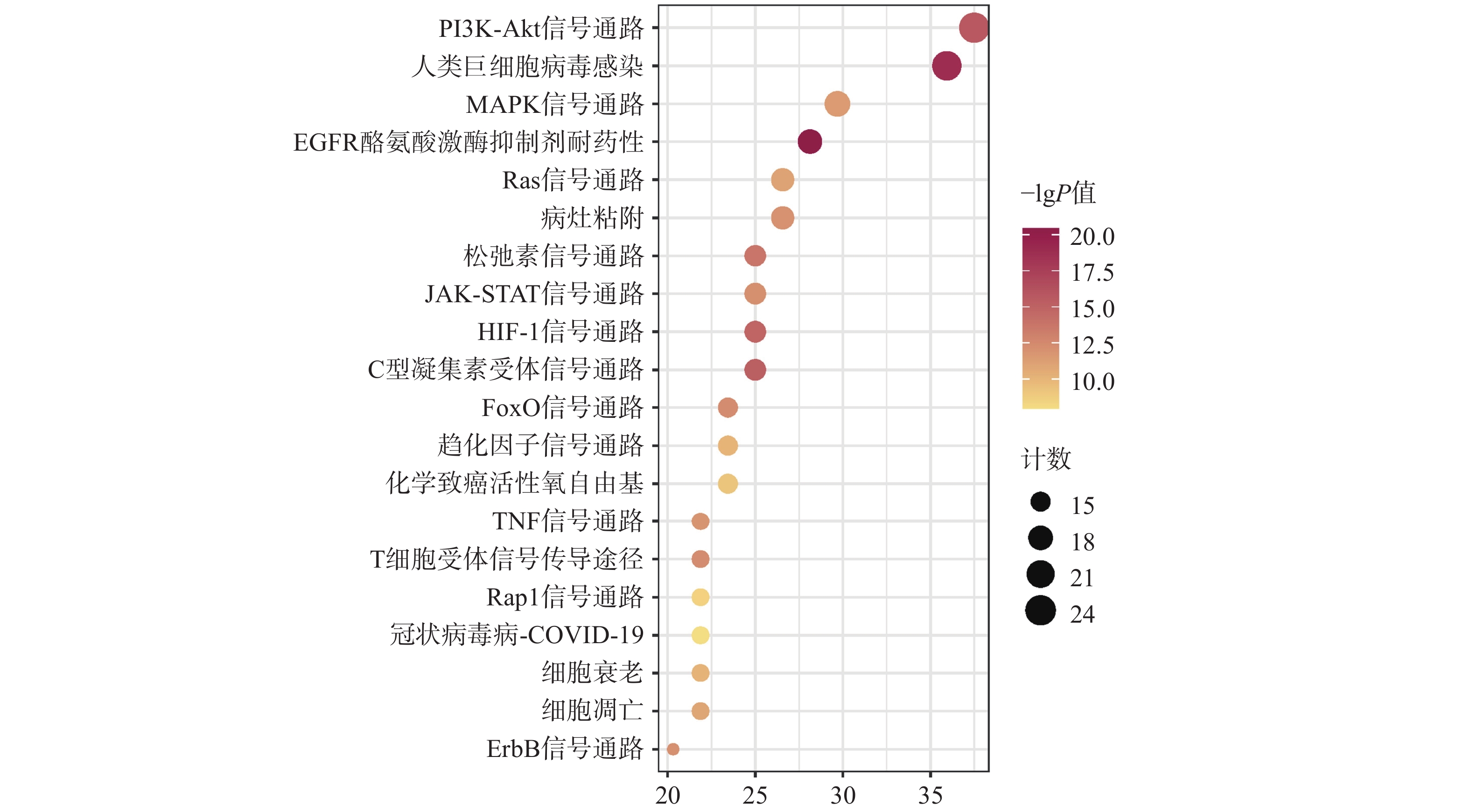
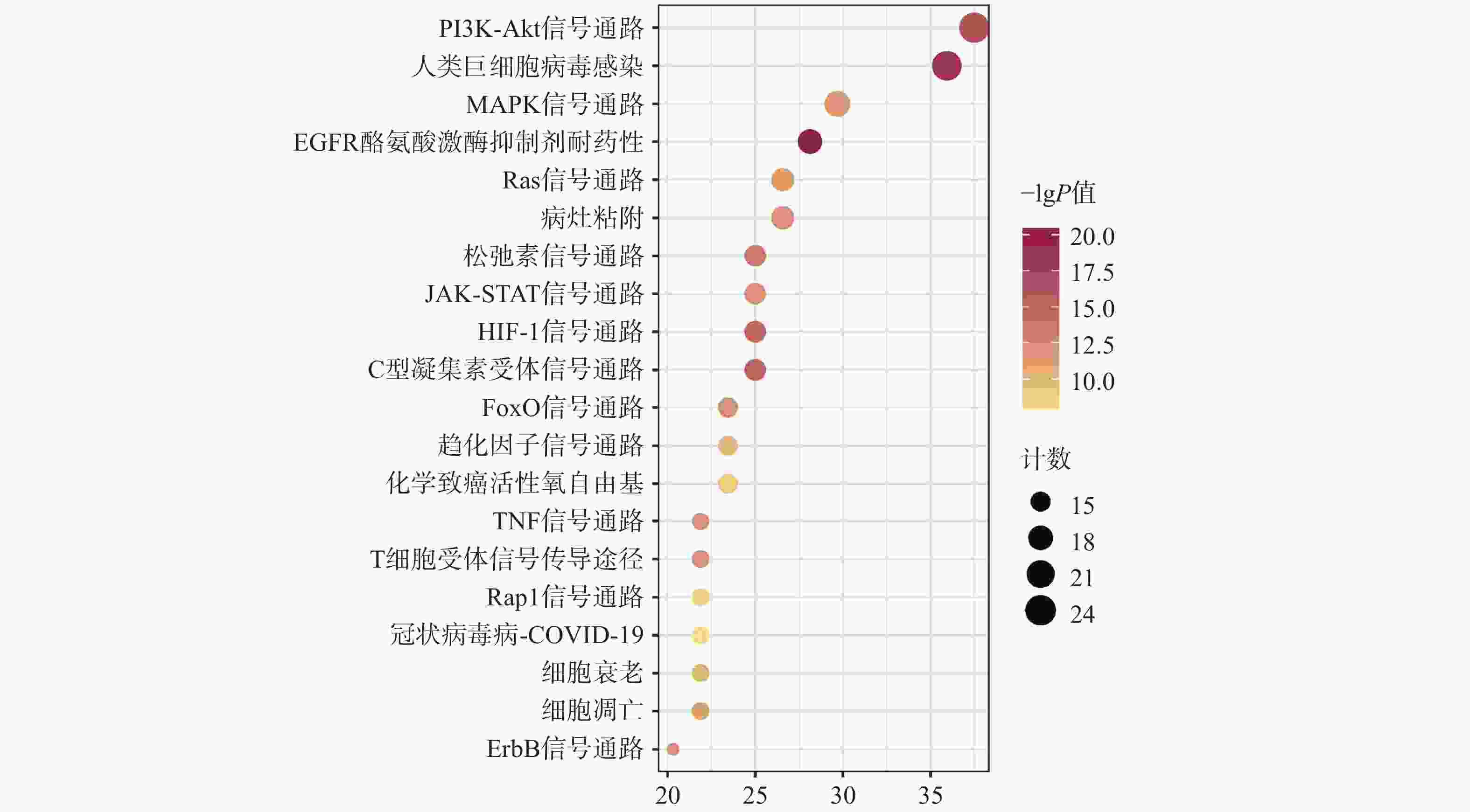
KEGG共富集到165条信号通路,选择与CAP密切相关的前20条,见图5。主要是PI3K/Akt信号通路、MAPK信号通路、趋化因子信号通路、TNF信号通路及细胞凋亡信号通路等。
-
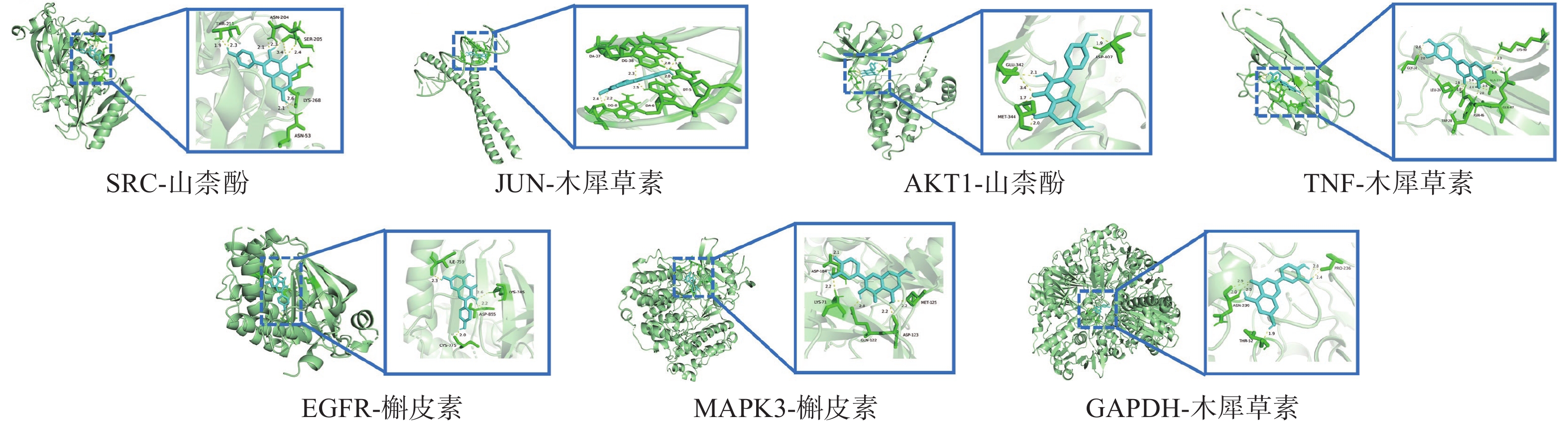
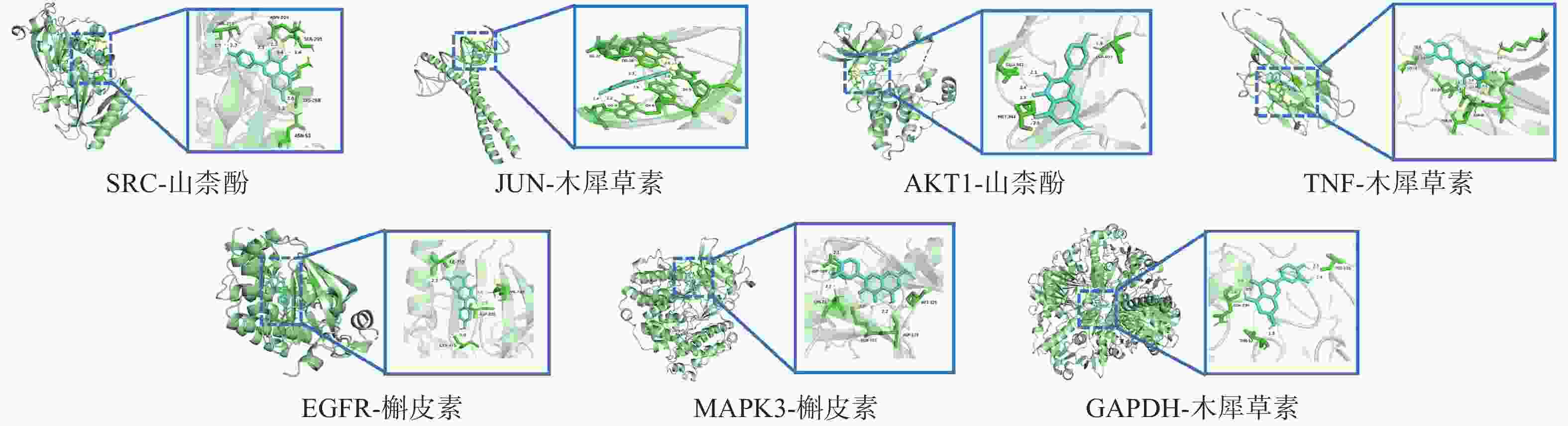
将“2.4”项中筛选得到部分核心靶点(GAPDH、AKT1、MAPK3、EGFR、TNF、SRC、JUN)与“2.1”项中筛选得到的部分核心成分(Quercetin, Kaempferol, Luteolin)进行分子对接。一般认为结合能<0 kJ/mol表明配体分子可以与受体分子自发结合,结合能<−20.92 kJ/mol则表示具有较好的结合能力,分子对接显示所有核心活性成分与关键靶点对接的结合能均<−20.92 kJ/mol,见表3。可视化分析结果见图6。
受体蛋白 PDB入口 配位基 亲和力(kJ/mol) GAPDH 8P5F 槲皮素 −30.79 山柰酚 −29.16 木犀草素 −30.92 AKT1 7NH5 槲皮素 −31.67 山柰酚 −32.47 木犀草素 −31.46 MAPK3 4QTB 槲皮素 −29.37 山柰酚 −27.36 木犀草素 −29.29 EGFR 8A2A 槲皮素 −34.56 山柰酚 −36.40 木犀草素 −32.05 TNF 5UUI 槲皮素 −26.94 山柰酚 −26.40 木犀草素 −27.32 SRC 7NG7 槲皮素 −28.45 山柰酚 −30.04 木犀草素 −28.66 JUN 5T01 槲皮素 −26.40 山柰酚 −26.28 木犀草素 −26.57 -
社区获得性肺炎的发展在很大程度上取决于宿主对气道中微生物的反应,失调的宿主反应因人而异,并对生存和其他结果产生负面影响,会诱导肺部局部和全身炎症反应[22]。有研究表明出院后的3个月高炎症标志物、死亡率也随之增加。因此,调节机体的免疫及炎症反应对治疗CAP是其关键之一[4]。
炎症是由不同病原体、刺激物或细胞损伤引起的血管组织中最重要的生物反应之一。被认为是生物体对抗病原体诱导的组织损伤的保护机制。在炎症过程中,吞噬细胞的活化会导致NADPH氧化酶的组装,急剧增加ROS的产生。内源性中和系统的活性饱和会导致ROS积累,同时,由于ROS的高化学反应性,会导致针对病原体和宿主生物系统的直接非特异性毒性。增加的ROS也可通过上调参与炎症反应的几个基因来引发和放大炎症。因此ROS通路可能成为炎症治疗的潜在靶点[23]。
本研究通过网络药理学方法,筛选后得到Degree值较高的靶点为GAPDH、AKT1、MAPK3、EGFR、SRC和TNF,这些靶点能通过调节炎症反应和ROS,在治疗CAP的过程中发挥重要作用。其中GAPDH能通过影响糖酵解,阻碍巨噬细胞活化,降低促炎细胞因子的产生来抑制炎症反应[24-25]。Akt是一种多功能激酶,通过调节血管通透性,导致水肿和白细胞外渗,影响炎症反应[26-27]。炎症相关基因的表达受到转录因子的严格调控,其中NF-κB是参与免疫和炎症反应的众多基因的多效性调节因子。氧化物质能导致其易位到细胞核中,通过上调各种促炎细胞因子和酶的产生来放大炎症反应,如白介素、TNF-α和诱导型NOS,增强ROS的细胞毒性作用。MAPK参与NF-κB 转录活性的调节,影响炎症反应和凋亡介质的合成。激活的EGFR也依赖于NF-κB信号传导途径,进一步调节促炎因子表达[28-30]。酪氨酸激酶(Src)作为NF-κB 和MAPKs的主要调节蛋白酪氨酸激酶之一,它可控制NADPH氧化酶活化和ROS产生,是ROS产生和细胞内稳态的主要调节因子,通过调节Src可抑制氧化应激及改善肺损伤中的炎症级联反应[31-33]。
GO及KEGG通路富集分析显示主要涉及蛋白结合、蛋白磷酸化、细胞凋亡及PI3K/Akt信号通路、MAPK信号通路等发挥关键作用。金芪清疏颗粒的核心活性成分主要为槲皮素、山柰酚、木犀草素,皆为黄酮类药物,具有广泛的抗炎、抗氧化、抗微生物作用。其中槲皮素通过激活细胞内MAPK通路,增加细胞内GSH水平,并在清除自由基反应中提供氢供体来源,从而提高细胞的抗氧化能力,抑制氧化应激[34]。还可通过清除NF-κB活化所必需的ROS,阻断TNF-α依赖的NF-κB核转移,降低IL-1β、IL-6、TNF-α水平和NF-κB表达来减轻早期炎症[23]。 此外还有研究表明槲皮素可减轻中性粒细胞气道炎症,抑制铁细胞凋亡和M1巨噬细胞极化[35]。山柰酚能减少ROS形成,抑制iNOS、环氧合酶和一氧化氮表达水平,降低炎症反应,并抑制MAPK途径的表达,减少趋化因子和IL-8的产生而降低炎症负荷[36-37]。木犀草素则能通过抑制NO及ROS产生、清除ROS和活化抗氧化酶、抑制促炎细胞因子表达、调节NF-κB通路、AKT和MAPK通路等途径发挥抗炎抗氧化作用[38-40]。在分子对接过程中核心靶点与核心活性成分均具有较好的结合能力,与当前研究相符。
综上所述,本研究通过网络药理学探讨了金芪清疏颗粒治疗CAP的作用机制,发现金芪清疏颗粒可能通过槲皮素、山柰酚、木犀草素等多种活性成分,作用多个疾病靶点,通过调控Akt、MAPK信号通路、改善氧化应激,发挥抗炎、抗氧化作用,从而明显改善肺部感染的炎症反应及肺部损伤,发挥保护作用。本研究证明金芪清疏颗粒具有多成分、多靶点、多通路的作用特点,为后续进一步深入研究其具体作用机制奠定基础。但仍缺乏相关实验验证,后续应进一步围绕氧化应激与炎症作用机制开展实验设计。
Study on the potential mechanism of JQQSG for the treatment of CAP based on network pharmacology and molecular docking technology
doi: 10.12206/j.issn.2097-2024.202312014
- Received Date: 2023-12-06
- Accepted Date: 2024-03-12
- Rev Recd Date: 2024-02-28
- Available Online: 2024-11-19
- Publish Date: 2024-11-25
-
Key words:
- JQQSG /
- community-acquired pneumonia /
- network pharmacology /
- inflammatory reaction /
- oxidative stress
Abstract:
| Citation: | CHEN Jintao, QIAO Ziying, MA Minghua, ZHANG Ruoxi, WANG Zhenwei, NIAN Hua. Study on the potential mechanism of JQQSG for the treatment of CAP based on network pharmacology and molecular docking technology[J]. Journal of Pharmaceutical Practice and Service, 2024, 42(11): 471-478. doi: 10.12206/j.issn.2097-2024.202312014 |







 DownLoad:
DownLoad: