-
胶质母细胞瘤(glioblastoma, GBM)是最常见的恶性脑肿瘤,具有高侵袭、高复发、预后差的特点,五年生存率27.3%[1]。由于肿瘤细胞弥漫性侵袭神经组织,通过手术难以做到完全切除,因而临床多采用多模式联合法结合放射治疗或化疗药物治疗[2]。肿瘤细胞的能量代谢具有高糖酵解率、高葡萄糖消耗、高乳酸堆积的特点,即Warburg效应。GBM等肿瘤细胞在氧气充足时,主要通过糖酵解快速获取能量,与三羧酸循环及氧化磷酸化途径获能的正常细胞相比,糖酵解率可高出20~30倍,并因此高消耗葡萄糖,用于产生足够能量以维持肿瘤细胞的生理活动与快速增殖[3]。因此Warburg效应是肿瘤细胞能量代谢的核心特征和主要驱动机制,同时参与肿瘤细胞重要的非代谢活动,如乳酸堆积参与GBM弱酸性微环境的形成,可促进GBM细胞的DNA修复过程[4]。葡萄糖氧化酶(Glucose oxidase, GOx)是一种天然氧化还原酶,能高效催化葡萄糖生成葡萄糖酸,大量消耗肿瘤细胞糖酵解所需葡萄糖。同时,GOx催化过程产生过氧化氢(H2O2),能中和肿瘤细胞产生的谷胱甘肽(GSH)以降低肿瘤细胞耐药性,造成肿瘤氧化损伤[5]。然而GOx具有体内易于失活、对正常组织产生毒性的特点,不利于应用。
纳米凝胶(nanogels, NGs)具有水凝胶和纳米颗粒的结构特征,粒径范围20~200 nm,可由蛋白质、多肽和多糖聚合物等天然大分子组成,经物理或化学方法形成三维的交联结构[6]。此外,基于物理交联的自组装特性,或二硫键、席夫碱等化学交联结构,纳米凝胶还表现出可调节的机械性能和对离子浓度、温度、和氧化还原剂等特定刺激的响应释药特性[7, 8]。透明质酸(hyaluronic acid, HA)是组成细胞外基质的主要成分之一,广泛分布于上皮、神经、结缔组织和肿瘤微环境中,具有良好的生物相容性。同时,HA可与CD44、CD168和细胞间粘附分子1(ICAM-1)等特异性细胞表面受体结合,实现肿瘤组织的靶向[9, 10]。
本研究合成了蛋氨酸修饰的HA两亲性聚合物HAM,与GOx交联构建的纳米凝胶GONGs。考察GONGs对胶质瘤细胞Warburg效应的抑制作用和对氧化损伤的诱导。
-
马尔文粒度电位仪(英国Malvern);透射电子显微镜(美国FEI);酶标仪、红外分光光度计、荧光分光光度计(美国Thermo)。高糖 DMEM 培养基、DMEM/F-12 培养基(中国源培);胰蛋白酶、青霉素-链霉素、DMSO(美国 Sigma);CCK8 试剂盒、Western blot试剂(中国碧云天); 辣根过氧化物酶标记山羊抗兔 IgG(H+L)(中国三鹰);兔重组单克隆抗体(中国华安);葡萄糖氧化酶(GOx, ≥250 U/mg, 中国Adamas life);透明质酸(HA, 35~50kDa)、Boc-L-蛋氨酸(Boc-L-Met)、胱胺二盐酸盐、1-乙基-(3-二甲基氨基丙基)碳酰二亚胺盐酸盐(EDCI)、N-羟基硫代琥珀酰亚胺(Sulfo-NHS)和京尼平购自中国源叶;PVDF 膜(美国 Millipore);小鼠胶质瘤细胞 GL261和人脐静脉内皮细胞细胞hUVEC购自中科院上海细胞库。
-
高糖DMEM培养基(10%FBS)培养GL261细胞(5% CO2、37 ℃),传至三代后接种至96孔板培养贴壁,设置不同分组给药:实验组为不同浓度GOx,不含GOx阴性对照组,空白培养基作为空白对照组(n=6)。给药孵育24 h后加入CCK8试剂孵育30 min,酶标仪检测吸光度值A,按以下公式计算细胞活力,绘制细胞活力曲线,计算IC50。
GL261细胞接种至12孔板。设置不同浓度GOx实验组与不加GOx对照组(n=3)。孵育24 h后提取细胞蛋白,Western Blot检测细胞中己糖激酶2(HK2)和L-乳酸脱氢酶(LDHA)的水平,以GAPDH为内参,因LDHA与GAPDH Western Blot条带位置相近,需在去除GAPDH抗体后再显示LDHA条带。
-
取HA、EDCI和sulfo-NHS溶于去离子水中,室温下搅拌反应2 h,加入胱胺反应24 h,去离子水中透析除去未反应物质,冻干得到中间产物HAN。取Met-boc、EDCI和sulfo-NHS溶于去离子水中,室温反应2 h,加入HAN反应24 h。产物HAM-boc透析后冻干得到HAM。取一定量的HA与HAM,红外吸收光谱法进行鉴定。
-
用PBS(pH 5.8, 0.1 mol/L)配制HAM溶液,磁力搅拌下加入一定质量比的GOx,搅拌30 min后再加入0.05%(w/v)的交联剂京尼平,混合液在 37 ℃下搅拌反应8 h,超滤离心除去游离GOx和京尼平,得到GONGs。
-
GOx溶解于PBS(pH8.5, 0.01 mol/L),FITC标记制备FITC-GOx,精确量取FITC-GOx,配制不同浓度的FITC-GOx标准溶液,荧光分光光度计测定荧光强度(Ex/Em, 488/520 nm),以荧光强度E与对照品浓度 C 作图,线性回归得标准曲线。
制备药载质量比1∶3、1∶5、1∶10、1∶15、1∶20的FITC-GONGs,超滤离心法分离FITC-GONGs和游离FITC-GOx,测定分离出的游离FITC-GOx荧光强度,计算FITC-GONGs的包封率与载药量,结合粒径确定最优投药比。包封率和载药量的计算公式为:
-
选择GOx和HAM药载质量比1∶10制备GONGs,马尔文激光粒度仪测定粒径、Zeta电位。取GONGs经醋酸铀负染,透射电镜观察粒径与外观形态。
-
DMEM/F-12培养基(10% FBS)培养hUVEC,接种于96孔板。配制不同浓度GONGs(GOx 0.4~102.4 nmol/L)作为实验组,同等浓度GOx作为对照组,各孔加入100 μL样品,孵育24 h,阴性对照组加入不含GOx培养基,空白培养基作为空白对照组(n=6)。按1.2中CCK8法测绘GOx组和GONGs组细胞活力曲线,计算并比较二组IC50,评估GONGs对hUVEC毒性。
-
新西兰兔(雄性,2.5 kg)静脉取血,离心分离法制备2.5%(v/v)的红细胞悬液。设置不同浓度GONGs(GOx 0.8~102.4 nmol/L)实验组,并配制同等浓度GOx对照组,生理盐水和TritonX-100分别作为阴性和阳性对照。各组样品与红细胞37 ℃孵育3 h,
3000 ×g离心10 min,观察各组上清液颜色。取100 uL上清于96孔板,酶标仪检测450nm处吸光度值,按如下溶血率公式计算各组溶血率,绘制GOx和GONGs的溶血率曲线: -
GL261接种至12孔板培养。设置不同浓度GONGs(GOx 1.0~4.0 nmol/L)实验组,不加GONGs的细胞作为对照组(n=3)。孵育24 h后提取蛋白,Western Blot检测HK2、LDHA、谷氨酸脱氢酶 1(GLUD1)和谷胱甘肽过氧化酶4(GPX4)蛋白的表达。
统计学分析方法均采用GraphPad Prism 8.3.0计算。细胞活力曲线绘制与IC50计算采用log(inhibitor)vs. response--Variableslope(four parameters)。数据比较采用 Unpaired Student’s T-test,P<0.05表示差异具有统计学意义。
-
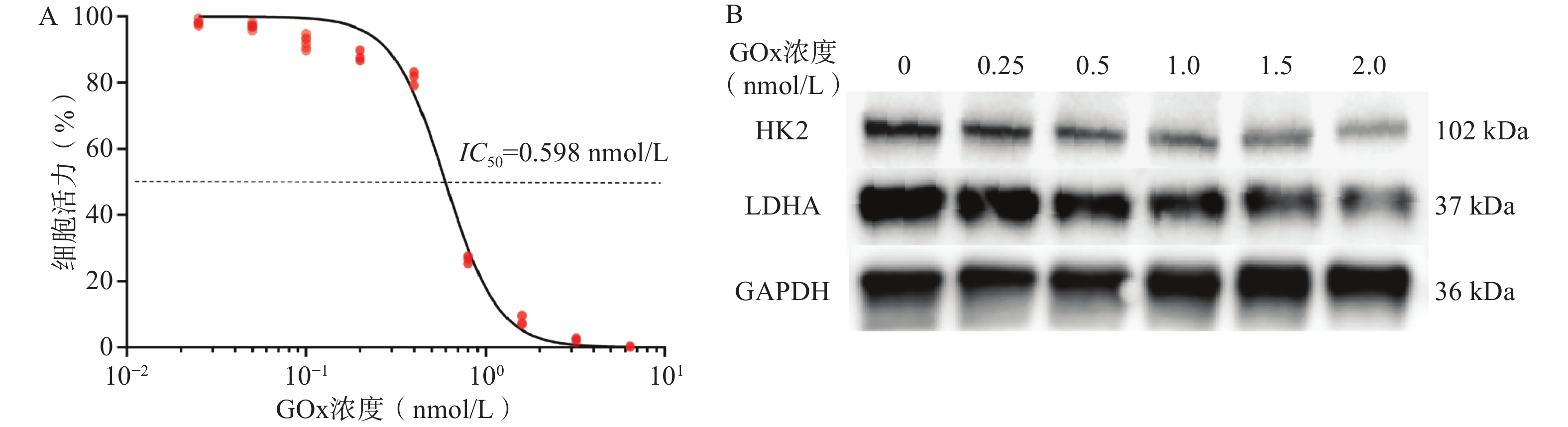
如图1A所示,GOx对GL261细胞24 h的IC50值为0.598 nmol/L(92.0 ng/mL),表明GOx对GL261细胞有较强的毒性。Western Blot结果如图1B所示,当GOx浓度为1.0 nmol/L时,GL261细胞HK2和LDHA的表达下调。其中,HK2是糖酵解途径的重要限速酶,LDHA是将糖酵解产物丙酮酸转化为乳酸并产能的关键酶,二者下调可初步验证GOx对GL261细胞Warburg效应有抑制效果。
-
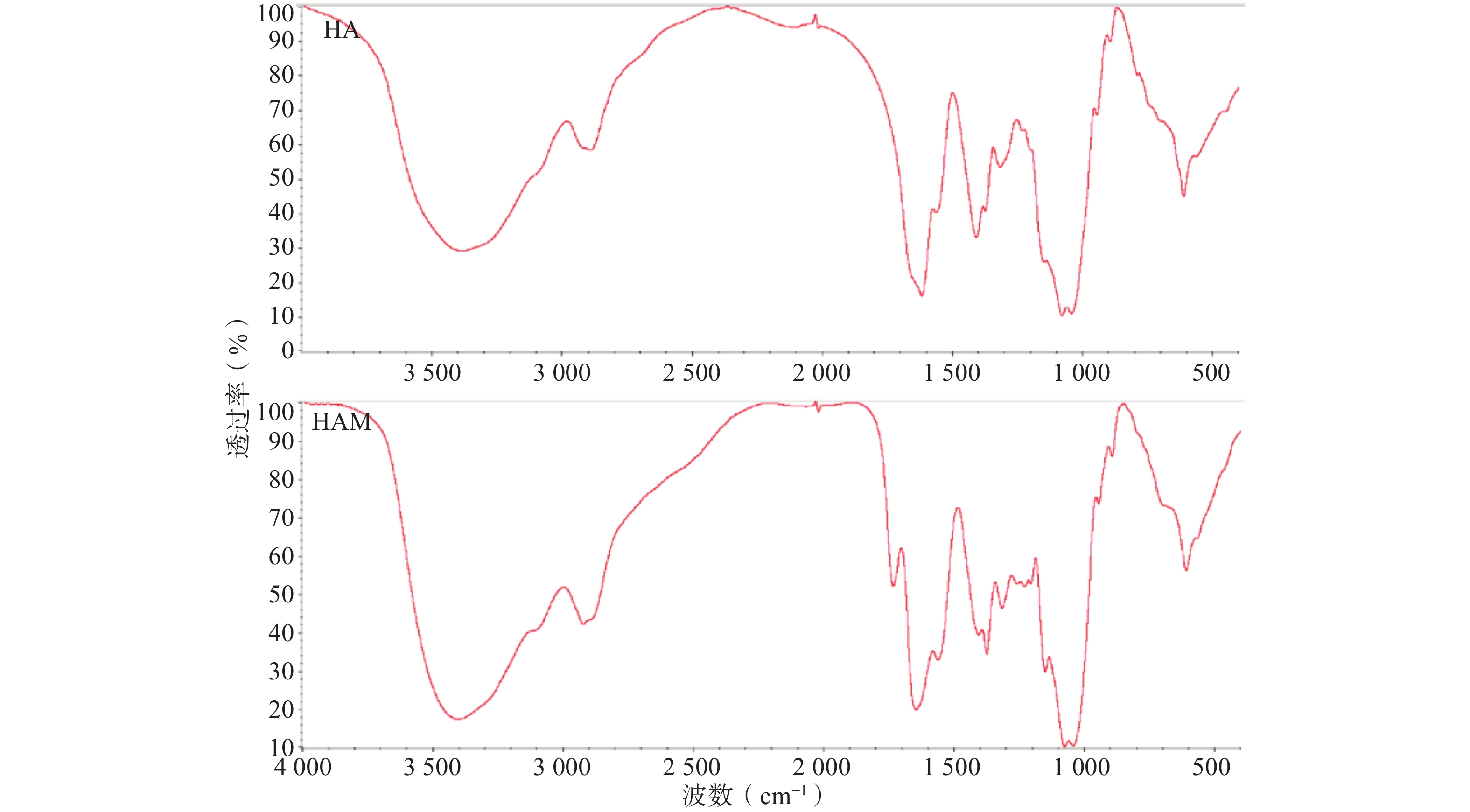
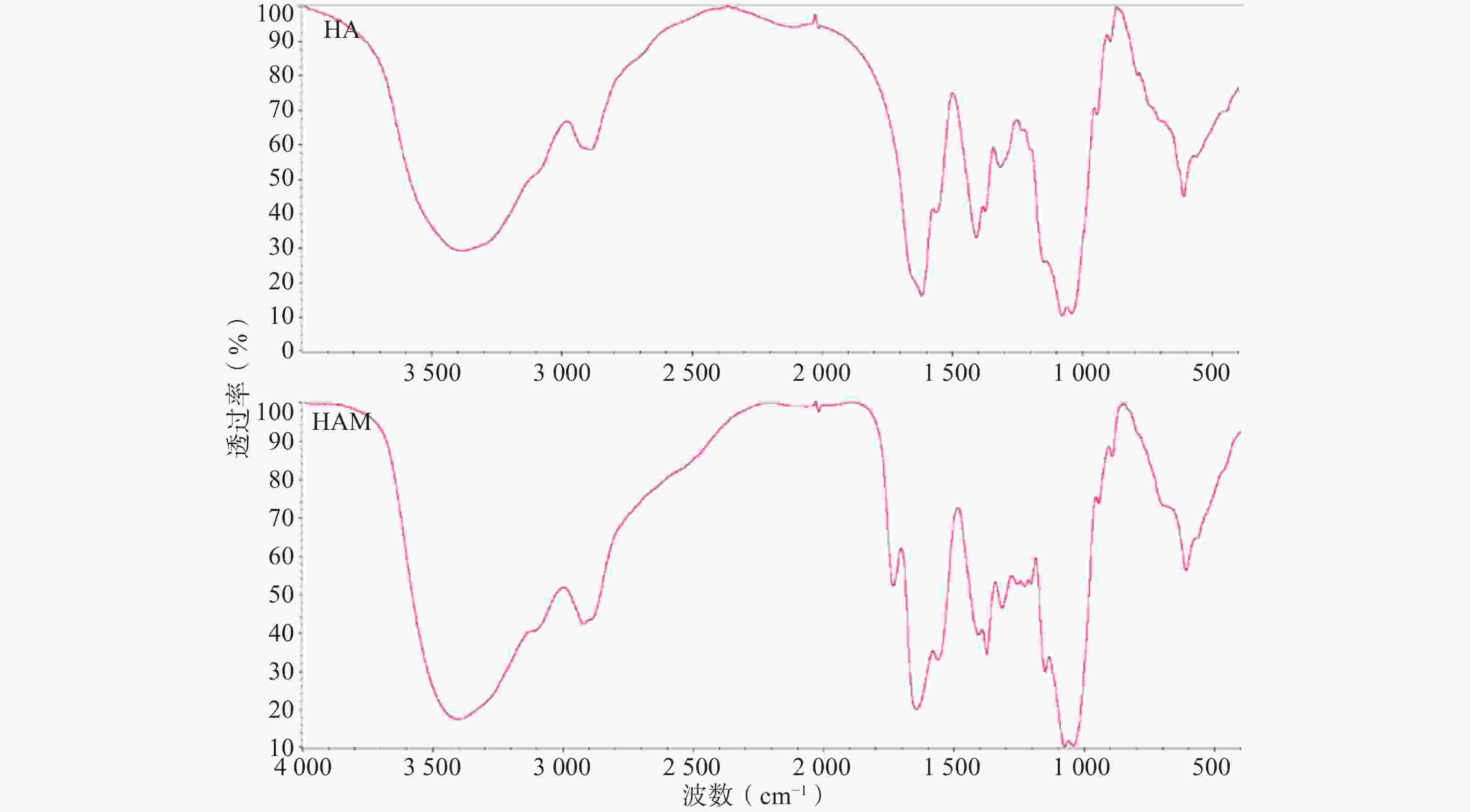
HA与HAM的红外投射光谱如图2所示,HAM的红外光谱相较HA在
1300 -1180 cm−1出现较明显吸收峰,应为反应连接的酰胺键C-O伸缩振动与蛋氨酸侧链硫甲基的弯曲振动作用重叠的结果。此外,1408 cm−1处吸收峰减弱应为HA部分羧基被反应,1738 cm−1吸收峰推测为部分质子化的羧基与酰胺键中羰基伸缩振动的作用结果。因产物经过充分透析,排除未连接的小分子Met与HA物理混合的可能,因此红外光谱结果可初步证明HAM的合成。 -
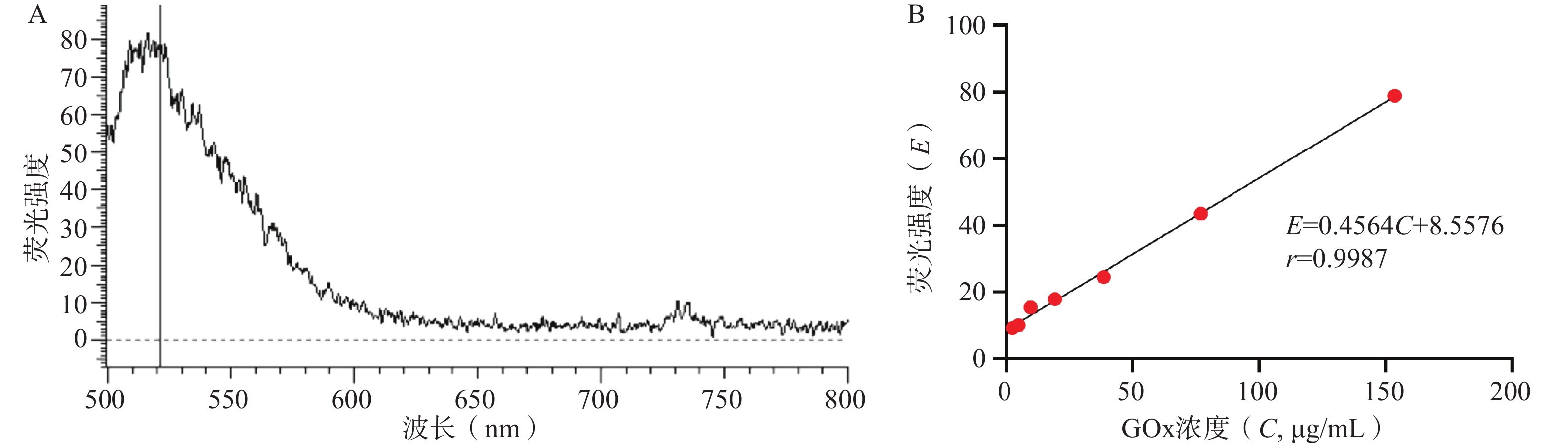
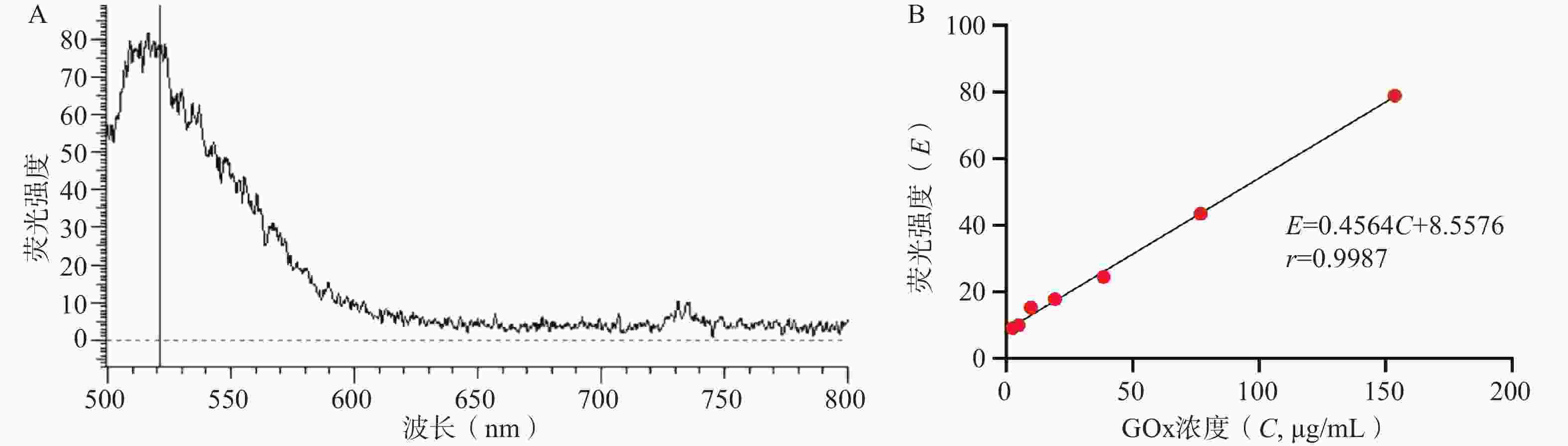
如图3所示,FITC-GOx在λEx=488 nm激发光下可见明显荧光,最大发射波长λEm=520 nm。在2.5~150 μg/mL的浓度范围内,GOx的浓度(C, μg/mL)与520nm处荧光强度(E)呈良好线性关系,线性回归方程为E=
0.4564 C+8.5576 ,r=0.9987 。GOx和HAM质量比对GONGs载药量、包封率和粒径的影响结果如表1所示,随着GOx比例升高,GONGs载药量逐渐增大。当投药比大于1∶10时,载体对GOx的包封率显著下降,且GONGs粒径降低至最小值,随后显著升高。HAM在pH5.8时,侧链氨基质子化不易自组装形成纳米颗粒,需要与正电性GOx共组装形成纳米凝胶GONGs。增加投料比时,过量的GOx引起纳米胶束聚集。因此选择GONGs载药量适中、包封率较高且粒径最小的1∶10投药比制备纳米胶束。
投药比(w/w) 载药量(%) 包封率(%) 粒径(nm) 1∶50 1.92±0.01 98.06±0.15 207.2±8.07 1∶20 4.64±0.01 97.37±0.26 193.2±11.06 1∶10 8.33±0.09 90.85±1.10 134.0±8.27 1∶5 13.57±0.24 78.18±1.63 201.9±13.47 1∶3 16.44±0.75 59.89±3.25 268.4±20.04 -
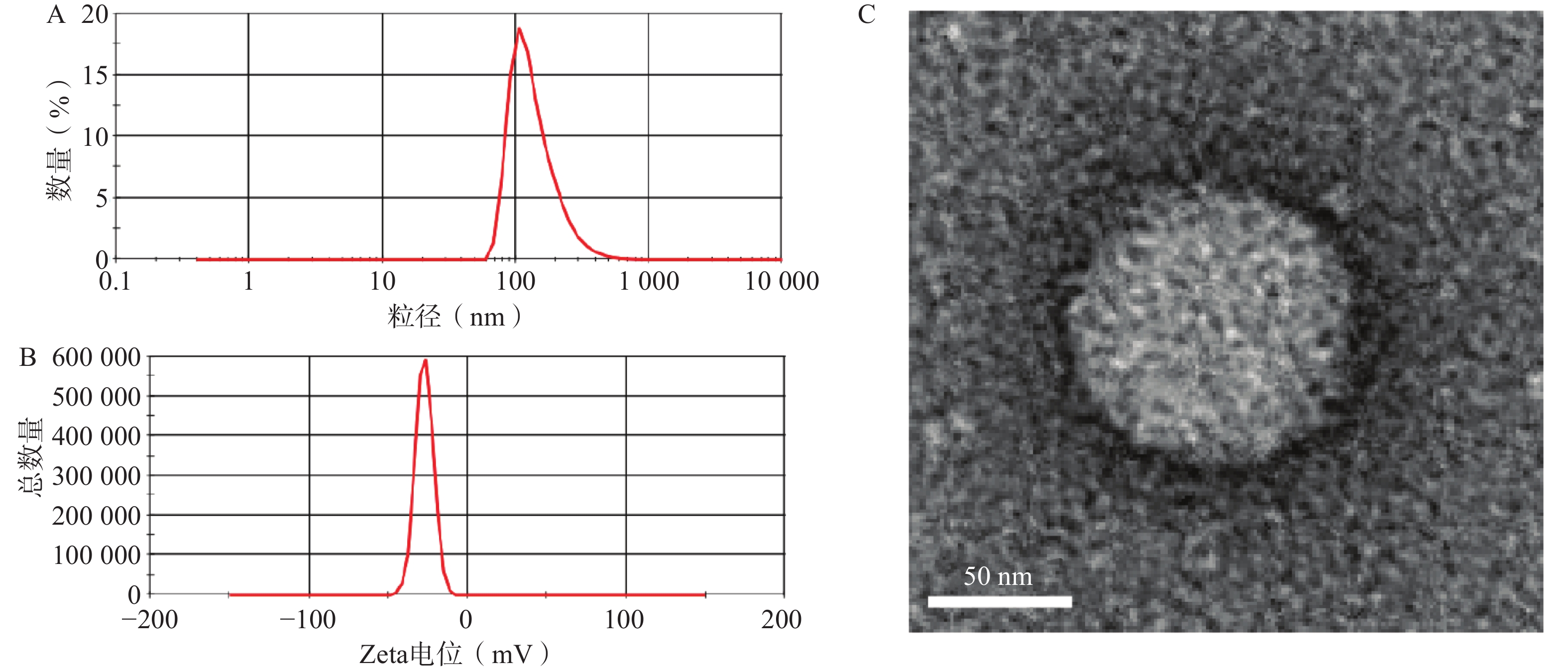
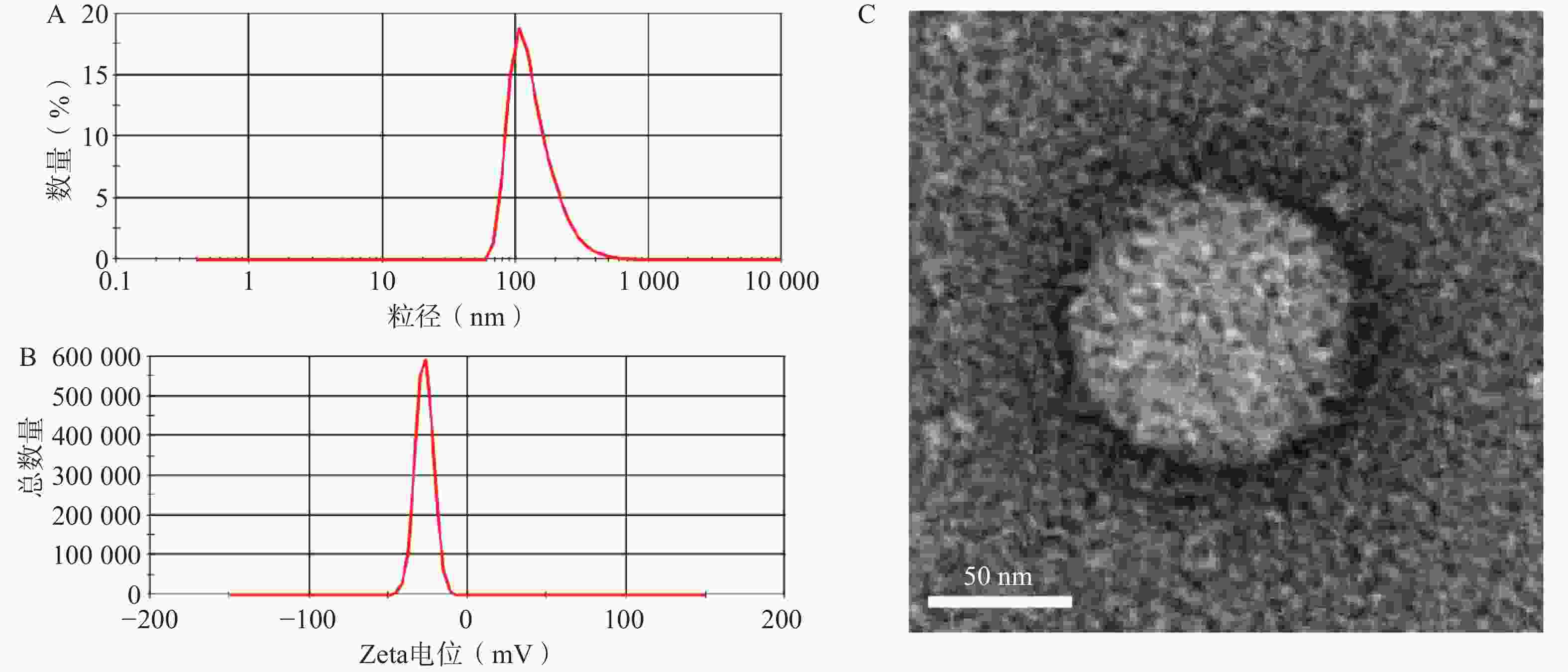
选择1∶10药载质量比制备GONGs并进行表征。如图4A、B所示,GONGs的粒径、Zeta电位分别为140.3±8.08 nm,−27.2±2.37 mV。投射电镜照片如图4C所示,GONGs粒子形状接近圆形,表明GONGs状态良好。
-
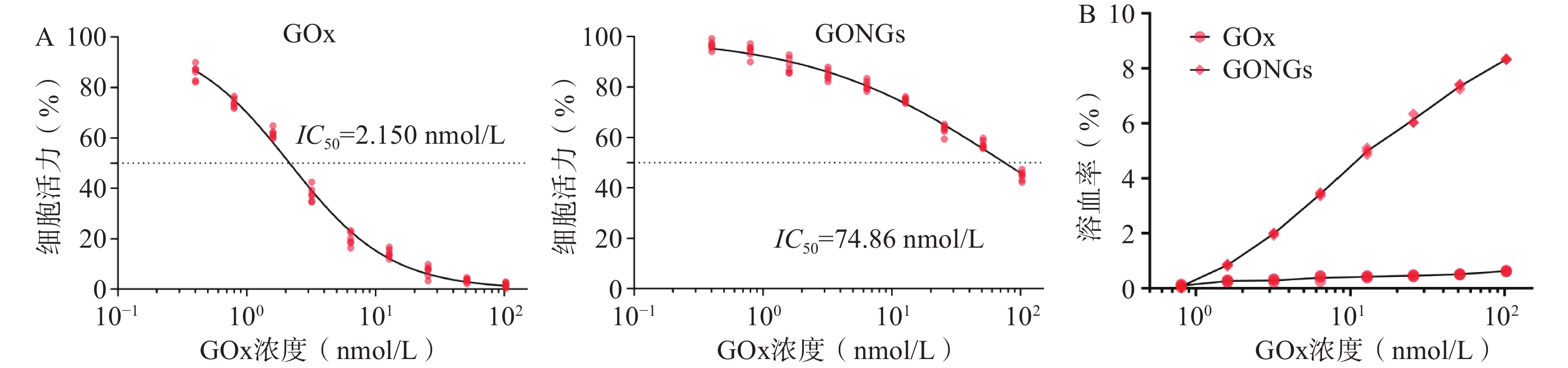
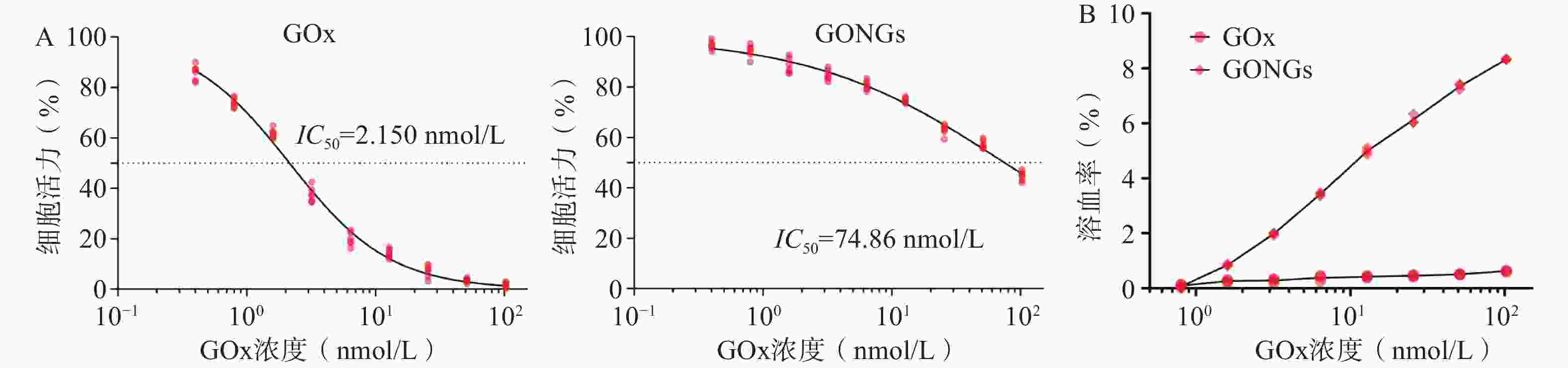
GOx与GONGs对hUVEC细胞增殖抑制效果如图5A所示, GONGs对hUVEC细胞的IC50为74.86 nmol/L,是GOx(2.150 nmol/L)的34.82倍,毒性显著降低。体外溶血实验结果如图5B所示,与红细胞悬液37 ℃恒温共孵育3 h后,GOx组随浓度上升出现少量溶血现象,而GONGs组体外溶血性较GOx显著减弱(P<0.01)。说明GONGs减少了药物对正常细胞的毒性,并抑制了溶血性。
-
如图6所示,GOx浓度为2.0 nmol/L时,GONGs给药后可见HK2和LDHA表达的下调,随着GONGs浓度升高而降低,表明GONGs能有效抑制GL261细胞的Warburg效应。此外,GL261细胞中GLUD1与GPX4表达量的显著降低,说明GONGs干预了胶质瘤细胞谷氨酰胺能量代谢途径, GOx催化过程中产生的H2O2对胶质瘤细胞造成了氧化损伤。
-
GBM作为恶性程度最高的高侵袭性原发脑肿瘤,存在复杂的病理机制,其预后极差且特征性地表现出增强的有氧糖酵解的Warburg效应,使得肿瘤细胞在氧气充足时仍通过糖酵解快速获取能量,在快速增殖与耐药机制中发挥重要作用。因此,针对肿瘤细胞Warburg效应抑制的治疗策略具有重要研究意义。GOx是一种天然氧化还原酶,可高效催化葡萄糖生成葡萄糖酸,大量消耗肿瘤细胞糖酵解所需葡萄糖抑制细胞产能,并产生大量H2O2作为ROS分子也可参与肿瘤的杀伤,也因此对正常细胞产生毒性。本文围绕大分子生物酶用于肿瘤糖酵解抑制,设计并构建了一种肿瘤微环境响应纳米凝胶GONGs,对于大分子活性成分,纳米凝胶结构可与之构成室状的负载结构,提高了GOx负载效率[11]。并利用载体的响应性释药和HA对肿瘤细胞的靶向递药减少正常组织的毒性作用。未来将进一步考察GONGs对GBM糖酵解代谢的作用机制以及药效学验证。
Construction of Glucose Oxidase–Loaded Nanogels and Its Inhibition Effect on the Warburg Effect in Glioma Cells
doi: 10.12206/j.issn.2097-2024.202511030
- Received Date: 2025-11-20
- Accepted Date: 2026-01-16
- Rev Recd Date: 2026-01-15
-
Key words:
- Glioblastoma /
- Nanogels /
- Glucose oxidase /
- Warburg effect
Abstract:
| Citation: | ZHOU Wenbo, LI Weilin, DAI Wuting, LIU Reiyao, YU Yuan. Construction of Glucose Oxidase–Loaded Nanogels and Its Inhibition Effect on the Warburg Effect in Glioma Cells[J]. Journal of Pharmaceutical Practice and Service. doi: 10.12206/j.issn.2097-2024.202511030 |








 DownLoad:
DownLoad: