-
肺癌是一种全球范围内发病率和死亡率均居高不下的恶性肿瘤 [1],根据病理类型及治疗策略的不同,可分为小细胞肺癌(SCLC)和非小细胞肺癌(NSCLC)。其中,NSCLC更为常见,约占所有肺癌病例的80%~85% [2]。
在驱动基因阳性的NSCLC治疗领域,靶向治疗凭借其精准性和高效性,已成为不可或缺的核心治疗手段。其中,表皮生长因子受体酪氨酸激酶抑制剂(EGFR-TKIs)作为一类靶向EGFR的小分子药物,与传统化疗相比,可显著改善EGFR突变阳性晚期NSCLC患者的生存获益,并有效延缓了耐药的发生,已被确立为一线治疗的标准策略 [3-4]。近年来,随着我国自主研发的第三代EGFR-TKIs(如阿美替尼、伏美替尼等)的相继上市,针对EGFR靶点突变的肺癌患者,临床上的治疗选择更加丰富,为患者提供了更多个性化的一线治疗方案。
与此同时,EGFR-TKIs的联合治疗方案也在不断探索与优化中。多项临床试验表明,部分联合治疗方案(如EGFR-TKIs联合抗血管生成药物或化疗)能够显著延长患者的生存获益,显示出优于单药治疗的潜力 [5-6]。然而,由于缺乏“头对头”的直接比较研究,不同治疗方案在疗效及安全性方面的差异尚未得到全面评估。基于此,本研究旨在通过网状Meta分析,系统评估EGFR-TKIs单药及其联合方案在晚期NSCLC一线治疗中的疗效与安全性,并通过概率排序为临床患者提供个体化治疗的循证医学依据。
-
本研究系统检索了PubMed、Embase、Cochrane Library数据库以及ClinicalTrials.gov网站的相关临床试验信息,同时补充检索了美国临床肿瘤学会(ASCO)、欧洲肿瘤内科学会(ESMO)和世界肺癌大会(WCLC)等国际会议摘要,以确保文献检索的全面性。检索时间范围为数据库建库至2023年6月30日,检索文献类型限定为随机对照试验(RCT),语言类型为英文。检索策略采用主题词与自由词相结合的方式,检索词包括“NSCLC”“EGFR-TKIs”“Randomized Controlled Trial”“Furmonertinib”“Gefitinib”“Dacomitinib”“Erlotinib”“Icotinib”“Afatinib”“Osimertinib”“Almonertinib”等。
-
①研究类型:Ⅱ期或Ⅲ期RCT。
②研究对象:年龄≥18岁,经组织学或细胞学确诊为EGFR突变阳性的晚期NSCLC患者,且既往未接受过EGFR-TKIs治疗。
③试验组:晚期EGFR突变NSCLC患者的一线治疗方案,包括EGFR-TKIs单药治疗或其联合治疗方案。
④对照组:EGFR-TKIs单药治疗或化疗。
⑤结局指标:疗效指标:总生存期(OS)、无进展生存期(PFS)、客观缓解率(ORR);安全性指标:3级及以上不良事件(≥3AEs)、严重不良事件(SAEs)。
-
①重复发表的研究。
②仅报告临床试验亚组数据的研究。
③无法获取完整数据的临床试验。
④动物实验、回顾性研究、综述、病例报告等。
-
由2名研究者根据预先制定的纳入和排除标准,独立完成文献筛选及数据提取工作。若在筛选或提取过程中出现分歧,将通过协商达成一致;若协商未果,则由第三方进行裁决。数据提取内容包括以下三部分:①研究基本信息:试验名称、试验阶段、发表年份、国家、试验注册号及纳入的结局指标;②患者基本信息:性别、年龄、样本量、试验组及对照组的治疗方案、肿瘤分期及EGFR突变类型;③临床指标结局:PFS、OS、ORR、≥3 AEs、SAEs。所有数据均从文献中的图表及文本中直接提取,若文献中缺失某些关键信息,则尝试联系原作者以获取补充数据。
-
采用《Cochrane系统评价员手册》(5.1.0版本)推荐的偏倚风险评估工具对纳入文献进行偏倚风险评估,评估内容包括以下7个条目:随机序列生成、分配隐藏、对患者及试验人员实施盲法、对结局评估者实施盲法、结局数据的完整性、选择性报告和其他偏倚。每个条目的偏倚风险分为低偏倚风险、偏倚风险不确定和高偏倚风险三个等级 [7]。由2名研究者根据评价标准独立完成评估,若出现分歧,则通过协商达成一致;若协商未果,则由第三方进行裁决。
-
使用Stata 17.0软件绘制网络证据图,以直观展示不同结局指标中治疗方案之间的直接比较与间接比较关系 [8]。在贝叶斯理论框架下,基于马尔科夫链蒙特卡洛(MCMC)方法,采用R软件(版本4.2.1)的“gemtc”包进行网状Meta分析 [9]。采用I²统计量评估研究间的异质性,若I2≥50%则认为存在高异质性,选择随机效应模型;否则,采用固定效应模型 [10]。
对于二分类变量(ORR、≥3 AEs、SAEs),效应量以比值比(OR)及其95%可信区间(CrI)表示;对于生存资料(PFS、OS),效应量以风险比(HR)及其95% CrI表示。通过累积排序概率图下面积(SUCRA)对治疗方案的优劣进行排序,SUCRA值范围为0%至100%,值越大表示该治疗方案获益越大 [11]。
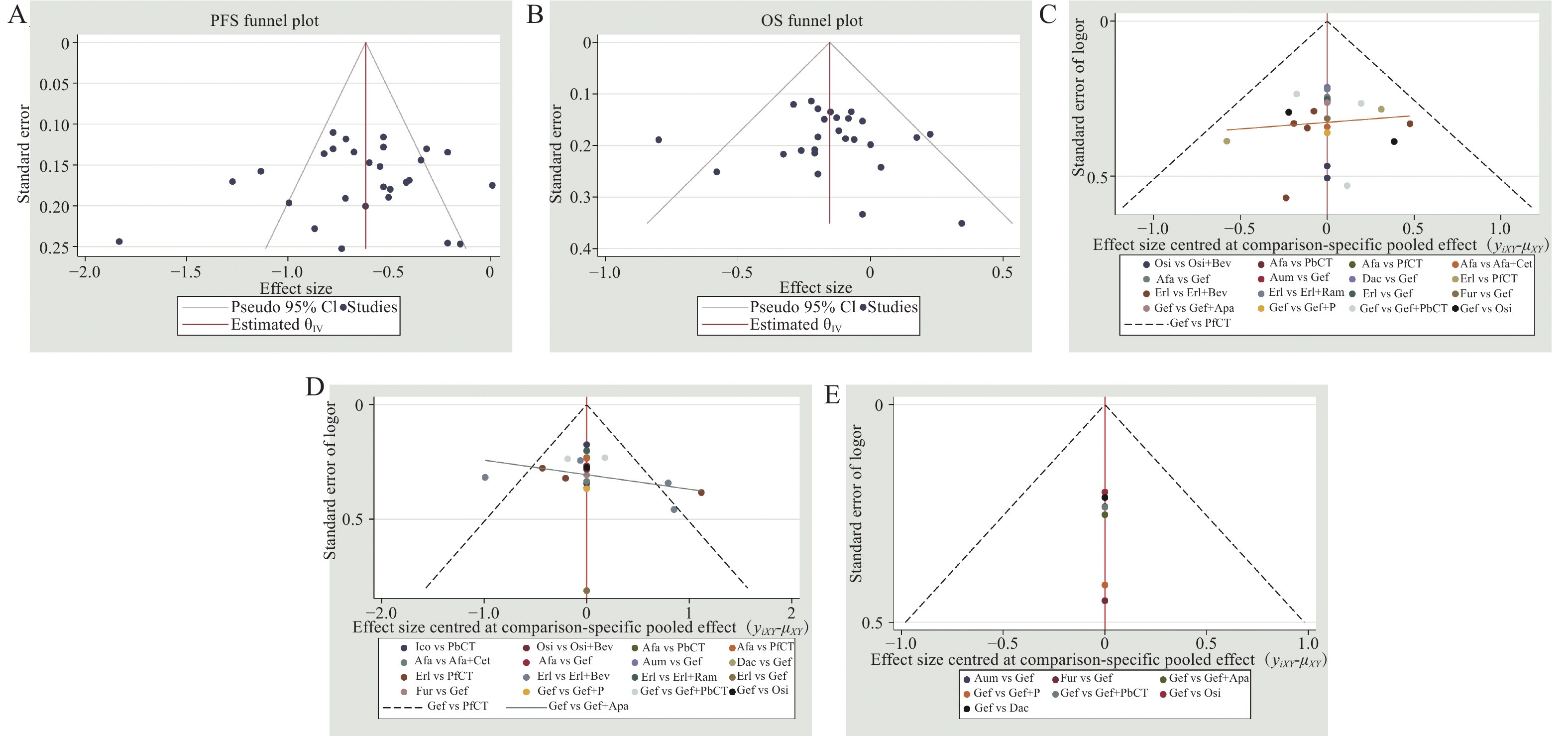
采用节点拆分法评估结果的局部不一致性 [12]。同时,根据患者的临床病理生理特征(如EGFR突变类型、是否为亚洲人群、是否存在脑转移、是否为吸烟人群、性别及年龄)对生存结局(OS、PFS)进行亚组分析,以探讨不同人群的疗效差异。最后,采用倒漏斗图评估发表偏倚及小样本效应 [13]。
-
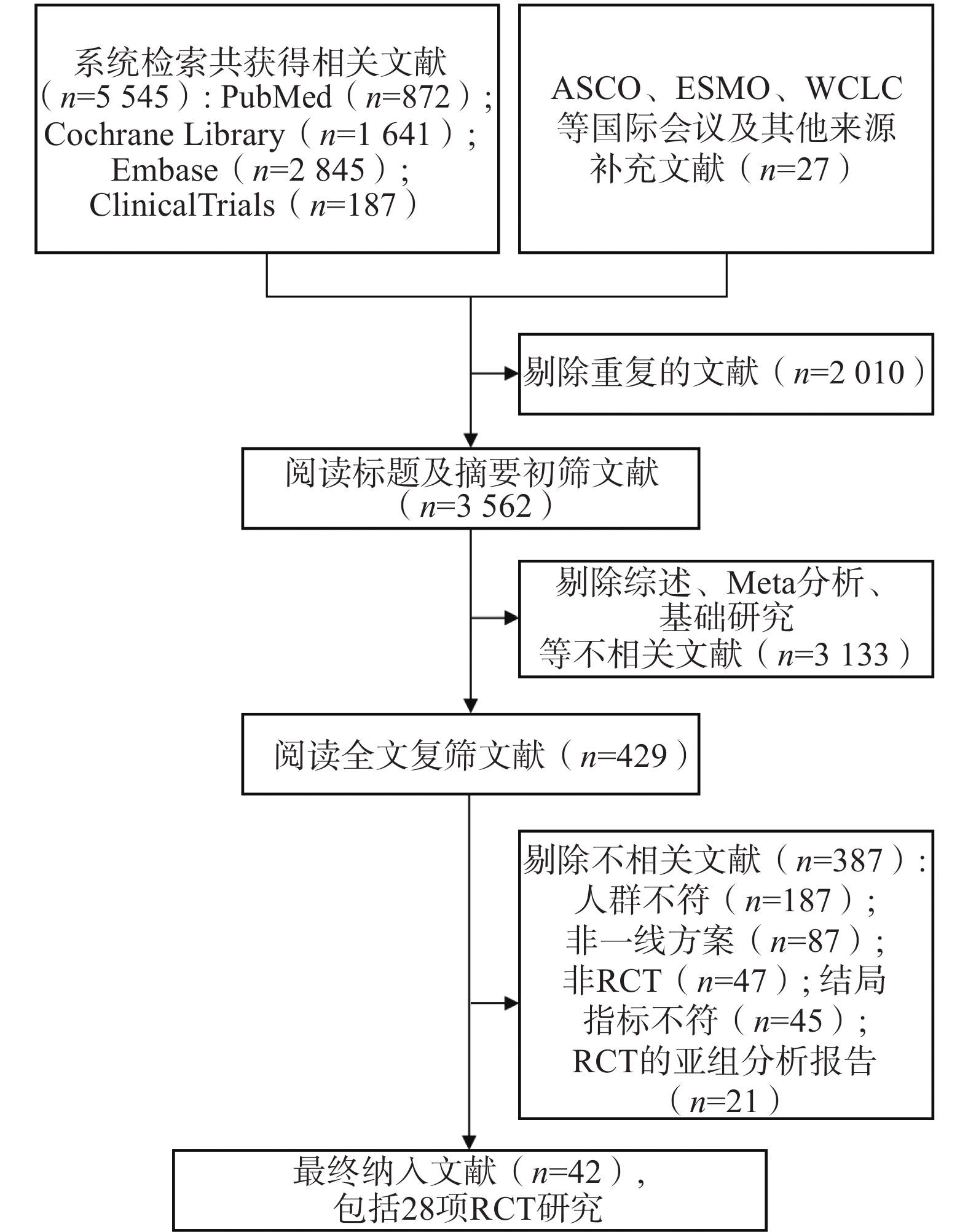
通过系统检索数据库及会议摘要,共获得5 572篇相关文献。剔除重复文献后,剩余
3562 篇;经过标题和摘要初筛,进一步剩余429篇文献。通过全文阅读复筛后,最终纳入28项RCT,文献筛选流程见图1。 -
28项RCT共纳入7 460例患者,其中试验组3 903例,对照组3 557例。研究涉及18种一线治疗方案,具体分类如下:①EGFR-TKIs单药治疗:吉非替尼、厄洛替尼、埃克替尼、阿法替尼、达可替尼、奥希替尼、阿美替尼、伏美替尼;②化疗:不含培美曲塞的化疗(PfCT)、含培美曲塞的化疗(PbCT);③EGFR-TKIs联合化疗:吉非替尼+培美曲塞、吉非替尼+含培美曲塞的化疗、埃克替尼+含培美曲塞的化疗;④EGFR-TKIs联合抗血管生成治疗:厄洛替尼+贝伐珠单抗、厄洛替尼+雷莫芦单抗、吉非替尼+阿帕替尼、奥希替尼+贝伐珠单抗;⑤EGFR-TKIs联合靶向药物:阿法替尼+西妥昔单抗。纳入研究的基本特征详见表1,各研究在试验设计及患者特征方面未见显著差异,认为研究间的比较具有良好的可传递性。
试验名称
(试验阶段,种群)实验组(T) 对照组(C) 样本量
(T/C)中位年龄
(岁)女性占比(%) 19Del
(T/C)L858R
(T/C)报告的结局 EGFR-TKIs单药对比化疗 WJTOG3405 2019
(Ⅲ,Asian) [14-15]吉非替尼 PfCT 86/86 64.0/64.0 68.6/69.8 50/37 36/49 PFS/OS/ORR NEJ002 2013
(Ⅲ,Asian) [16-17]吉非替尼 PfCT 114/110 63.9/62.6* 63.2/64.0 58/59 49/48 PFS/OS/ORR/
≥3AEsOPTIMAL 2015
(Ⅲ,Asian) [18-19]厄洛替尼 PfCT 82/72 57.0/59.0 58.5/59.7 43/39 39/33 PFS/OS/≥3AEs/SAEs EURTAC 2012
(Ⅲ,non-Asian) [20]厄洛替尼 PfCT 86/87 65.0/65.0 67.4/78.2 57/58 29/29 PGS/OS/ORR/≥3AEs/SAEs LUX-Lung3 2015
(Ⅲ,multiple) [21-22]阿法替尼 PbCT 230/115 61.5/61.0 63.9/67.0 113/57 91/47 PFS/OS/ORR/≥3AEs LUX-Lung6 2015
(Ⅲ,Asian) [21-23]阿法替尼 PfCT 242/122 58.0/58.0 64.0/68.0 124/62 92/46 PFS/OS/ORR/≥3AEs ENSURE 2015
(Ⅲ,Asian) [24]厄洛替尼 PfCT 110/107 57.5/56.0 61.8/60.7 57/61 52/46 PFS/OS/ORR/≥3AEs/SAEs CONVINCE 2017
(Ⅲ,Asian) [25]埃克替尼 PbCT 148/137 56.0/56.0 70.9/69.3 80/74 68/63 PFS/OS/≥3AEs LUX-Lung7 2017
(Ⅱ,multiple) [26-27]阿法替尼 吉非替尼 160/159 63.0/63.0 57.0/67.0 93/93 67/66 PFS/OS/ORR/≥3AEs CTONG0901 2017
(Ⅲ,Asian) [28]厄洛替尼 吉非替尼 128/128 NG 53.1/53.9 74/74 54/54 PFS/OS/ORR/≥3AEs ARCHER1050 2021
(Ⅲ,multiple) [29-31]达可替尼 吉非替尼 227/225 62.0/61.0 64.3/55.6 134/133 93/92 PFS/OS/ORR/≥3AEs/SAEs FLAURA 2020
(Ⅲ,multiple) [32-33]奥希替尼 吉非替尼/
厄洛替尼279/277 64.0/64.0 63.8/62.1 175/174 104/103 PFS/OS/ORR/≥3AEs/SAEs AENEAS 2022
(Ⅲ,Asian) [34]阿美替尼 吉非替尼 241/215 59.0/62.0 62.6/62.8 140/141 74/74 PFS/OS/ORR/≥3AEs/SAEs FURLONG 2022
(Ⅲ,Asian) [35]伏美替尼 吉非替尼 178/179 59/60 65.2/62.0 91/92 87/87 PFS/OS/ORR/≥3AEs/SAEs EGFR-TKIs联合化疗/免疫/靶向对比EGFR-TKIs单药 JMIT 2020
(Ⅱ,Asian) [36-37]吉非替尼+培美曲塞 吉非替尼 126/65 62.0/62.0 65.0/63.0 65/40 52/23 PFS/OS/ORR/≥3AEs/SAEs Hanetal 2020
(Ⅱ,Asian) [38-39]吉非替尼+PbCT 吉非替尼 40/41 NG 62.5/56.1 21/21 19/20 PFS/OS/ORR Xu et al 2019
(Ⅱ,Asian) [40]埃克替尼+PbCT 埃克替尼 90/89 58.6/61.0* 63.3/74.2 51/52 38/37 PFS/OS/ORR Noronha et al 2020
(Ⅲ,Asian) [41]吉非替尼+培美曲塞 吉非替尼 174/176 54.0/56.0 49.2/47.2 107/109 NG PFS/OS/ORR/≥3AEs/SAEs NEJ009 2020
(Ⅲ,Asian) [42-43]吉非替尼+PbCT 吉非替尼 170/172 64.8/64.0* 67.1/62.8 93/95 69/67 PFS/OS/ORR/≥3AEs JO25567 2014
(Ⅱ,Asian) [44]厄洛替尼+贝伐珠单抗 厄洛替尼 75/77 67.0/67.0 60.0/66.2 40/40 37/37 PFS/OS/ORR/≥3AEs/SAEs NEJ026 2020
(Ⅲ,Asian) [45]厄洛替尼+贝伐珠单抗 厄洛替尼 112/112 67/68 63.4/65.2 56/55 56/57 PFS/OS/ORR/≥3AEs/SAEs Stinch et al 2019
(Ⅱ,non-Asian) [46]厄洛替尼+贝伐珠单抗 厄洛替尼 43/45 65/63 68.9/72.1 30/29 15/14 PFS/OS/ORR RELAY 2019
(Ⅲ,multiple) [47]厄洛替尼+雷莫芦单抗 厄洛替尼 224/225 65/64 62.9/63.1 123/120 99/105 PFS/OS/ORR/≥3AEs/SAEs CTONG1706 2021
(Ⅲ,Asian) [48]吉非替尼+阿帕替尼 吉非替尼 157/156 57/60 58.0/6.3 81/83 74/73 PFS/OS/ORR/≥3AEs/SAEs ARTEMIS 2021
(Ⅲ,Asian) [49]厄洛替尼+贝伐珠单抗 厄洛替尼 157/154 57/59 61.8/62.3 82/79 75/75 PFS/ORR/OS/≥3AEs WJOG9717L 2022(Ⅱ,Asian) [50] 奥希替尼+贝伐珠单抗 奥希替尼 61/61 67/66 60.7/62.3 35/36 26/25 PFS/OS/ORR/≥3AEs BEVERLY 2022
(Ⅲ,non-Asian) [51]厄洛替尼+贝伐珠单抗 厄洛替尼 80/80 65.9/67.7 65.0/62.5 44/44 34/32 PFS/OS/ORR/≥3AEs/SAEs SWOGS1403 2020
(Ⅱ,multiple) [52]阿法替尼+西妥昔单抗 阿法替尼 83/85 66.5/66.3 71.1/62.4 53/54 30/31 PFS/OS/ORR/≥3AEs T/C:试验组/对照组;19Del:EGFR 19外显子缺失;L858R:EGFR 21外显子L858R突变;PfCT:不含培美曲塞的化疗;PbCT:含培美曲塞的化疗;PFS:无进展生存期;OS:总生存期;ORR:客观缓解率;≥3 AEs:3级及以上不良事件;SAEs:严重不良事件;NG:无数据;*:无中位年龄数据,使用平均年龄代替。 -
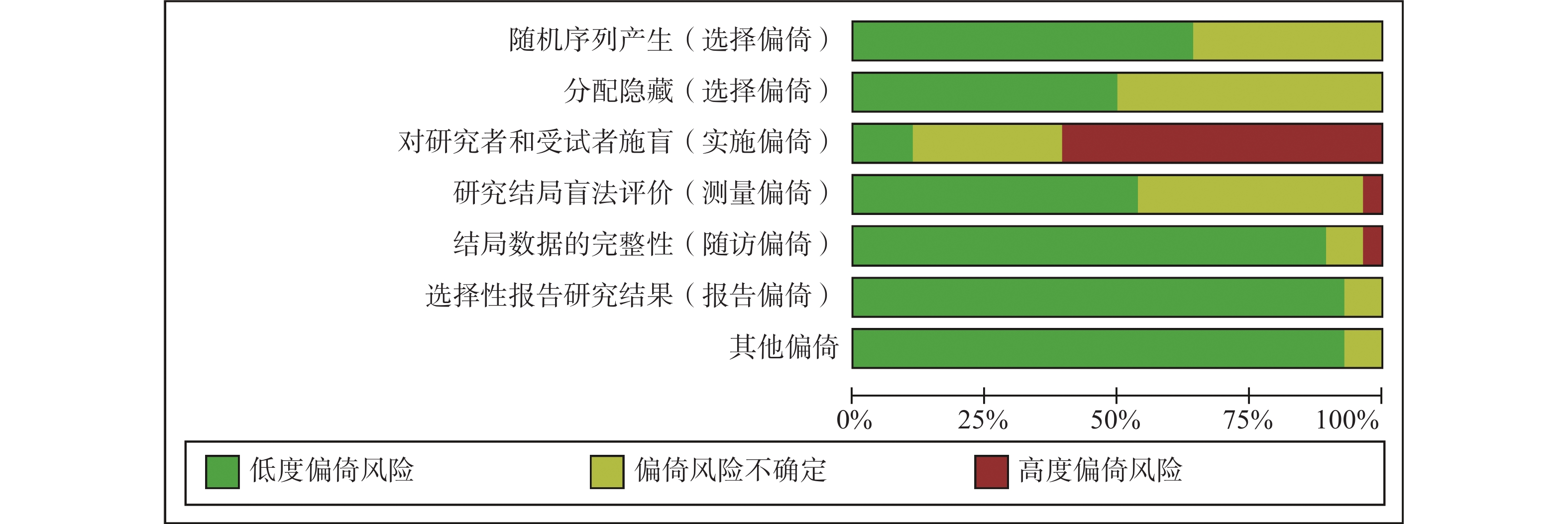
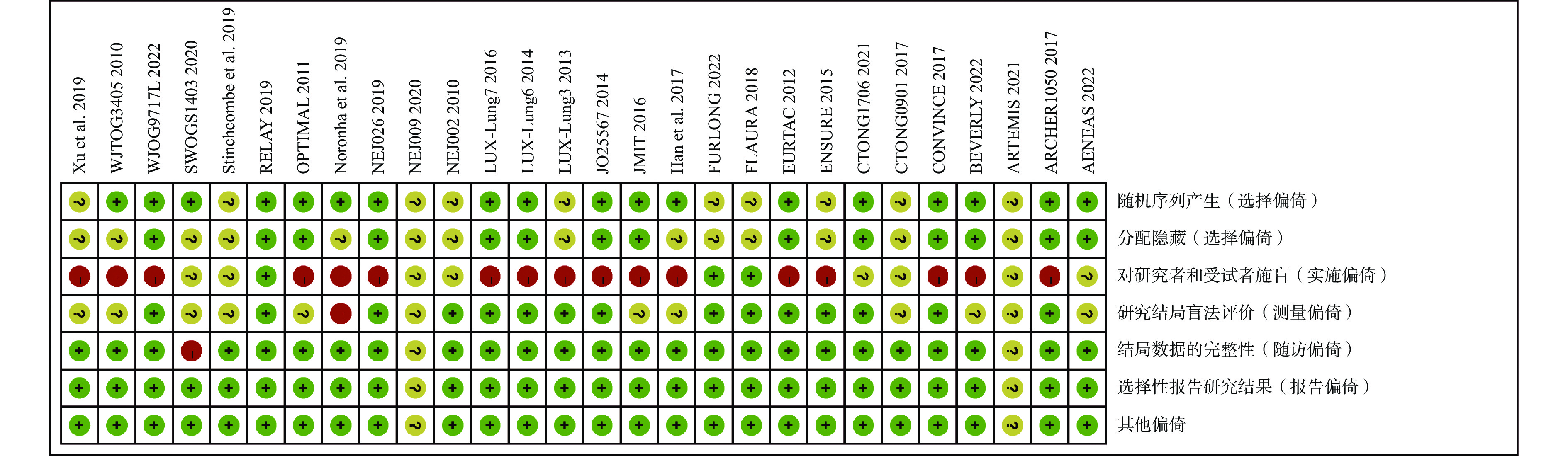
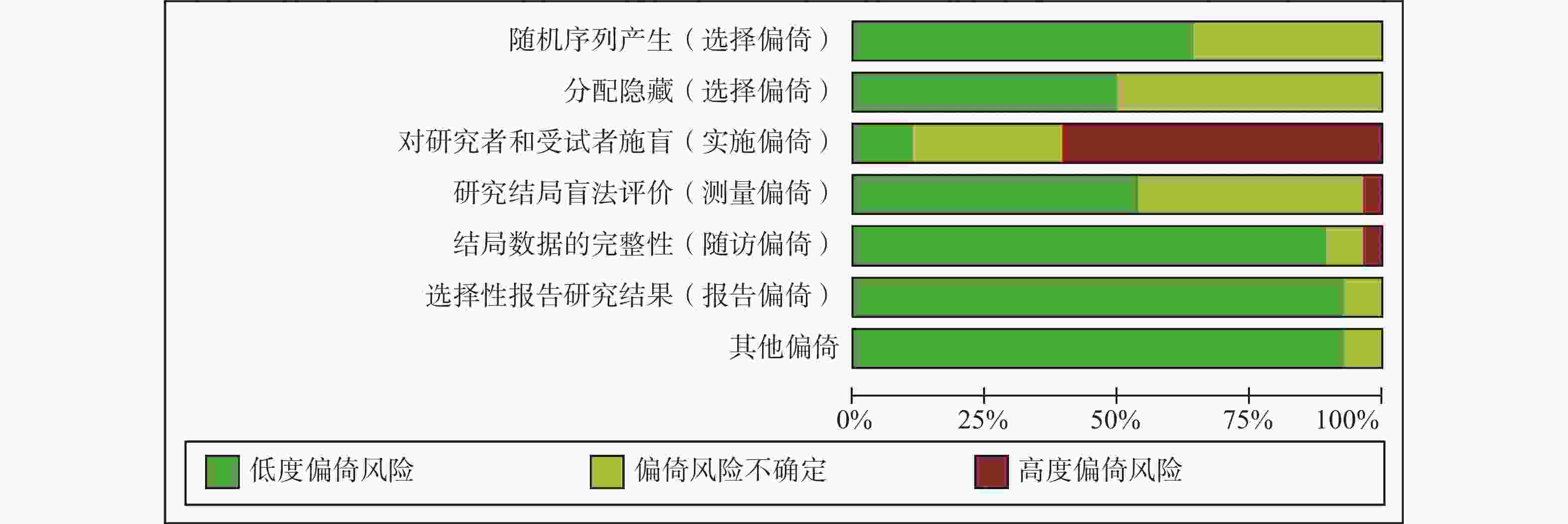
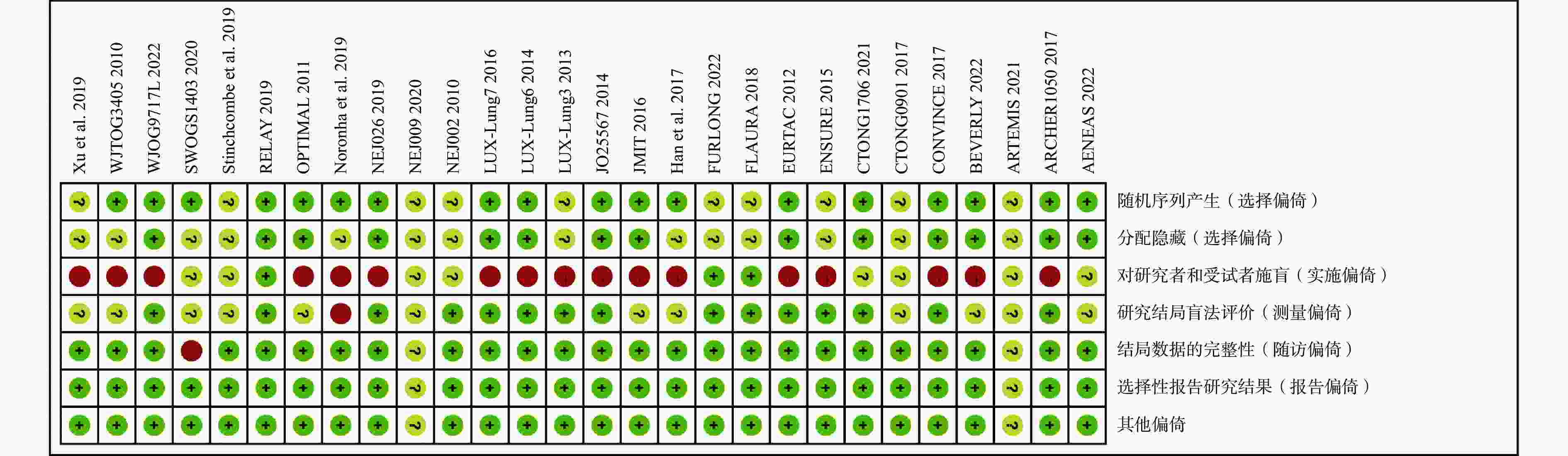
采用Cochrane偏倚风险评估工具对纳入的28项RCT进行偏倚风险评估,结果见图2和图3。评估结果显示,纳入研究的整体偏倚风险较低,在“实施偏倚”条目下,部分研究因涉及口服与静脉用药方案的比较,难以对研究者和患者实施盲法,导致该条目被评估为高风险。除此之外,其他条目下大多数研究均为低偏倚风险。
-
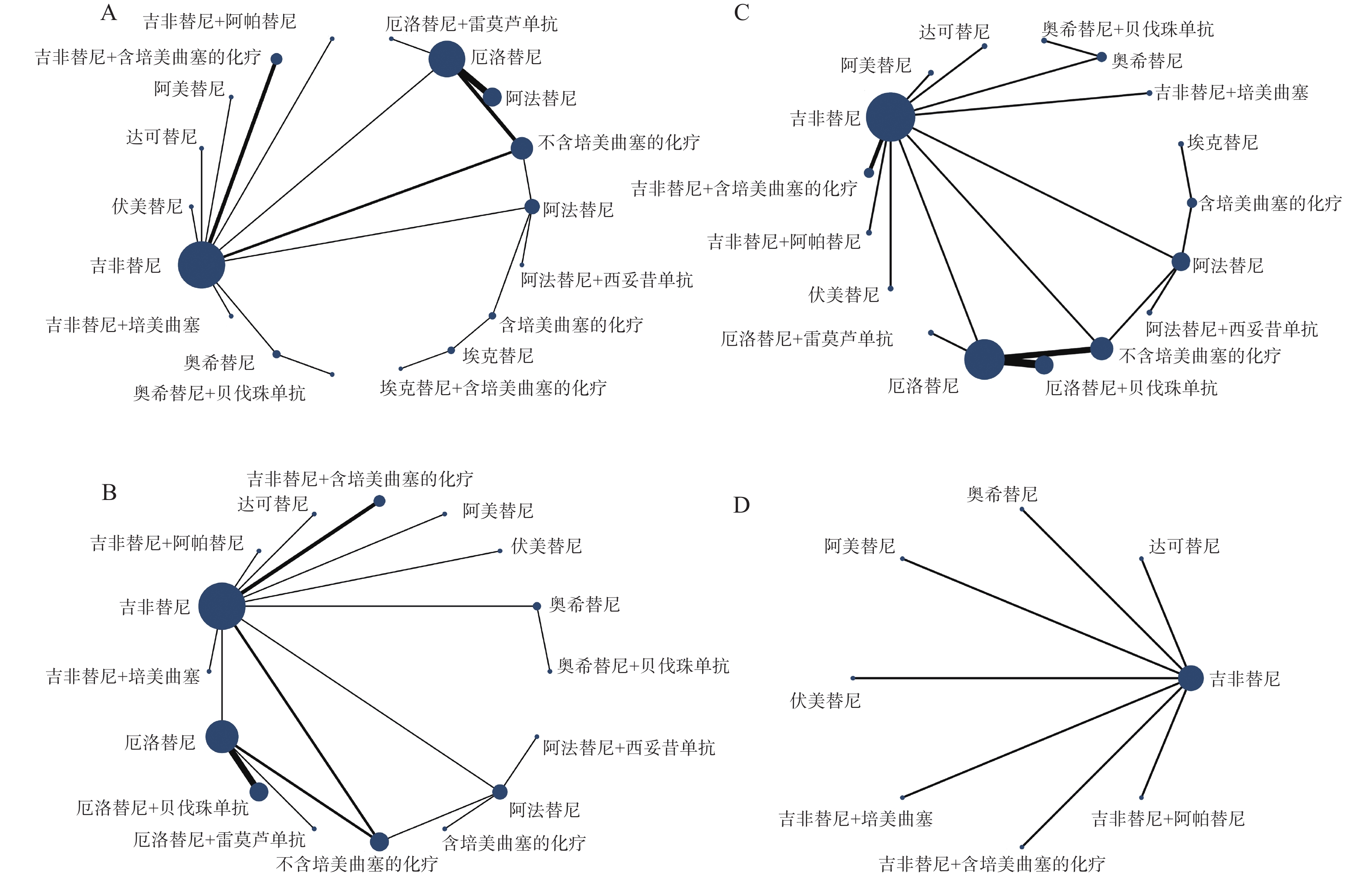
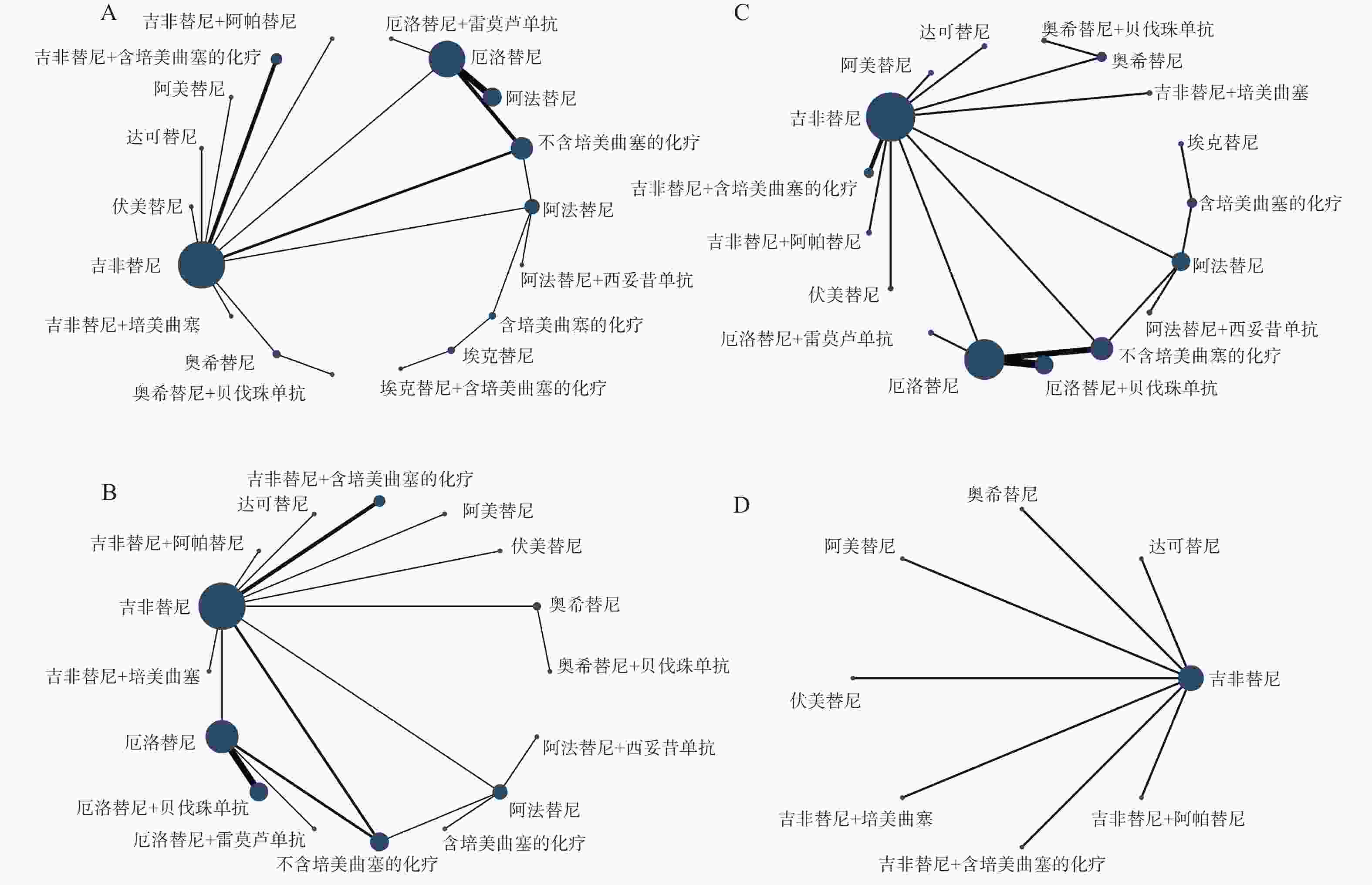
各结局指标的网络证据图见图4,具体情况如下:PFS与OS纳入18种一线治疗方案进行比较(图4A);ORR纳入16种一线治疗方案进行比较(图4B);≥3AEs纳入17种一线治疗方案进行比较(图4C);SAEs纳入8种一线治疗方案进行比较(图4D)。
-
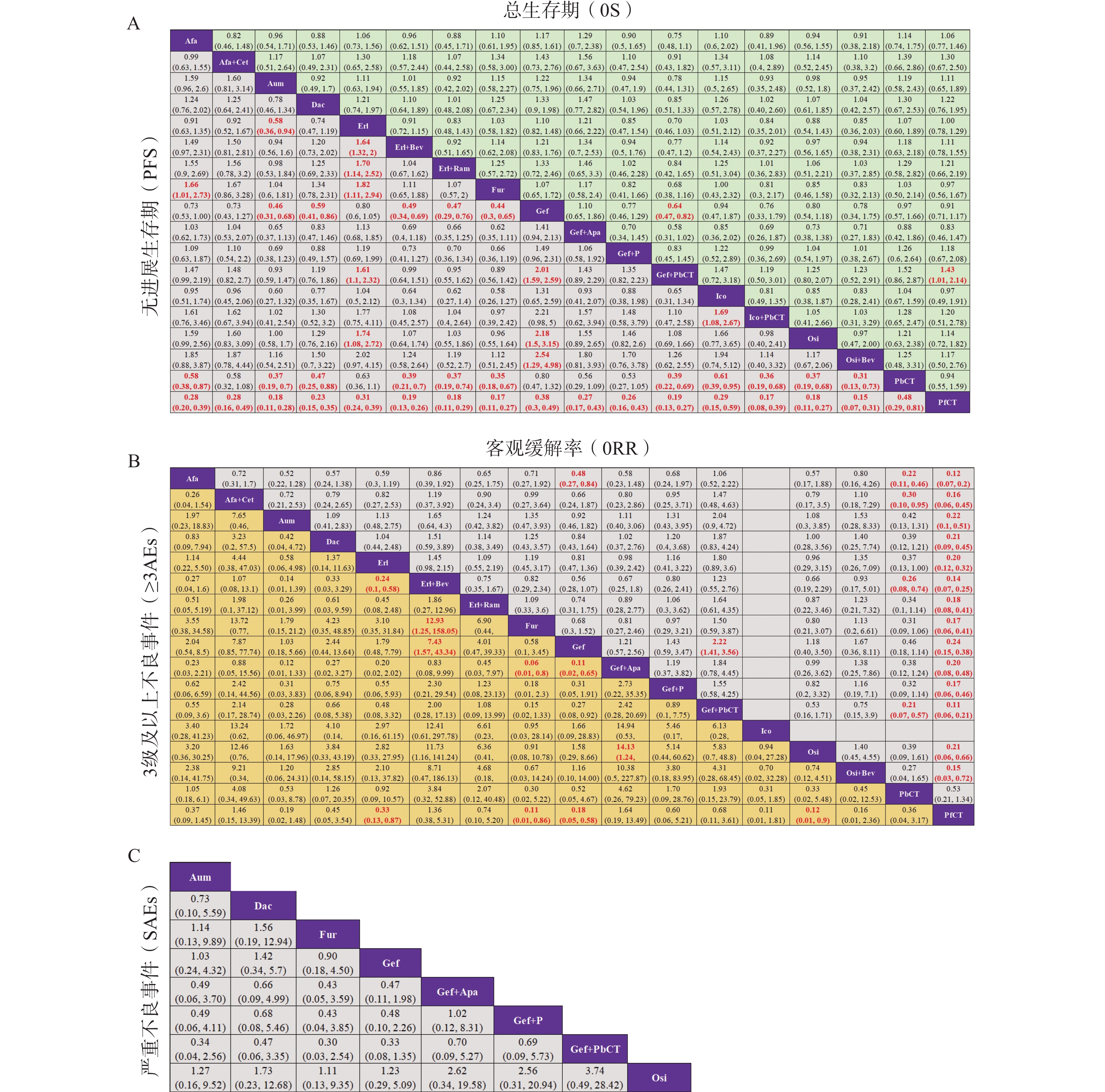
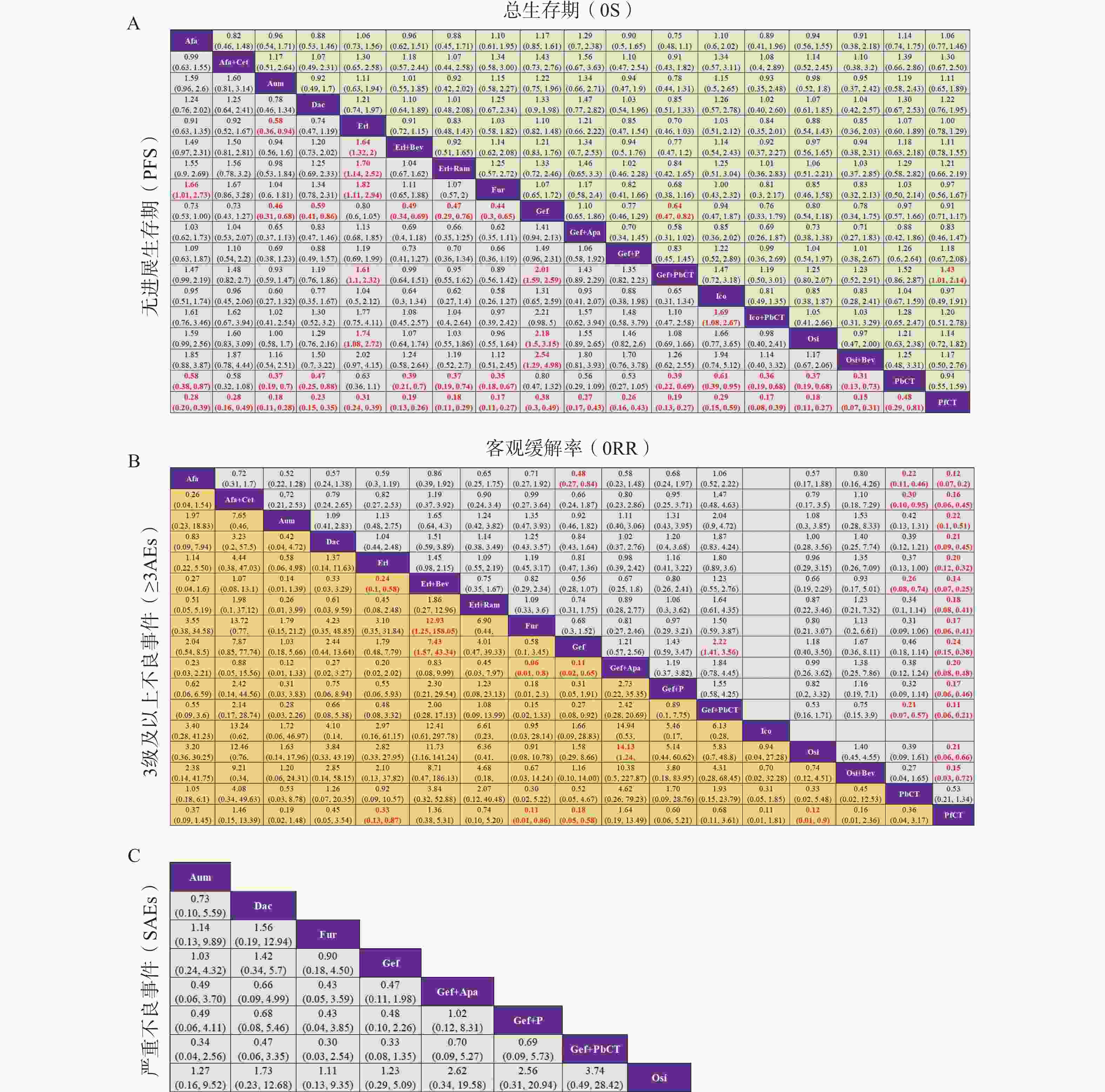
基于异质性检验结果(I² > 50%),本研究采用随机效应模型进行统计分析。不同治疗方案在OS、PFS、ORR、≥3AEs及SAEs等主要结局指标下的两两比较结果见图5,其中,治疗方案间存在显著性差异(P < 0.05)的结果以红色加粗字体标注。在OS方面,吉非替尼联合含培美曲塞的化疗相较于吉非替尼单药或不含培美曲塞的化疗,显示出显著的生存获益;而在SAEs方面,纳入分析的8种一线治疗方案之间均未观察到显著性差异。此外,通过节点拆分法进行的不一致性检验结果显示,各结局指标下的P值均 > 0.05,表明直接证据与间接证据之间具有良好的一致性。
-
不同治疗方案在各结局指标下的SUCRA值及排名见表2。在纳入分析的干预措施中,各结局指标排名首位的治疗方案分别为:①PFS:奥希替尼+贝伐珠单抗(SUCRA=83.08%);②OS:吉非替尼+含培美曲塞的化疗(SUCRA=84.38%);③ORR:吉非替尼+含培美曲塞的化疗(SUCRA=85.72%);④≥3AEs:伏美替尼(SUCRA=83.54%);⑤SAEs:奥希替尼(SUCRA=75.91%)。
治疗方案 缩写 PFS OS ORR ≥3AEs SAEs 奥希替尼+贝伐珠单抗 Osi+Bev 83.08(1) 56.67(8) 63.34(4) 71.77(5) NA 伏美替尼 Fur 80.36(2) 37.09(15) 59.38(6) 83.54(1) 68.25(2) 阿美替尼 Aum 76.80(3) 53.59(10) 39.67(13) 70.64(6) 63.62(4) 奥希替尼 Osi 76.64(4) 57.30(7) 46.71(9) 82.04(2) 75.91(1) 埃克替尼+含培美曲塞的化疗 Ico+PbCT 74.49(5) 60.15(6) NA NA NA 厄洛替尼+雷莫芦单抗 Erl+Ram 74.33(6) 61.87(4) 53.87(8) 34.16(12) NA 厄洛替尼+贝伐珠单抗 Erl+Bev 71.71(7) 54.34(9) 73.78(3) 16.32(16) NA 吉非替尼+含培美曲塞的化疗 Gef+PbCT 70.33(8) 84.38(1) 85.72(1) 34.12(13) 16.28(8) 达可替尼 Dac 53.80(9) 65.35(3) 44.92(12) 46.43(10) 47.64(5) 吉非替尼+培美曲塞 Gef+P 43.20(10) 60.21(5) 57.26(7) 38.39(11) 30.94(6) 吉非替尼+阿帕替尼 Gef+Apa 38.18(11) 21.75(18) 46.17(11) 15.24(17) 29.58(7) 阿法替尼+西妥昔单抗 Afa+Cet 36.34(12) 68.26(2) 59.66(5) 18.12(15) NA 阿法替尼 Afa 35.75(13) 48.99(11) 82.33(2) 52.48(9) NA 埃克替尼 Ico 34.52(14) 37.74(14) NA 79.73(3) NA 厄洛替尼 Erl 28.41(15) 38.37(13) 46.40(10) 57.14(7) NA 吉非替尼 Gef 13.83(16) 23.37(17) 30.48(14) 74.37(4) 67.79(3) 含培美曲塞的化疗 PbCT 8.18(17) 32.11(16) 9.65(15) 52.71(8) NA 不含培美曲塞的化疗 PfCT 0.05(18) 38.47(12) 0.67(16) 22.79(14) NA NA:无数据,未纳入排名。 -
针对不同临床病理生理特征的人群,不同治疗方案在PFS及OS结局中的亚组分析结果见表3和表4。
治疗方案 所有人群 突变 种族 吸烟 性别 脑转移 年龄 19Del L858R 亚洲 非亚洲 是 否 男性 女性 是 否 ≥65岁 <65岁 奥希替尼+贝伐珠单抗 1 1 12 4 2 11 4 4 3 2 伏美替尼 2 3 5 2 10 4 3 7 5 1 5 1 阿美替尼 3 5 9 3 13 1 8 2 1 5 2 6 奥希替尼 4 6 4 8 1 7 5 9 3 4 3 1 5 埃克替尼+含培美曲塞的化疗 5 14 1 9 1 10 5 6 厄洛替尼+雷莫芦单抗 6 4 7 6 4 3 1 5 10 2 厄洛替尼+贝伐珠单抗 7 2 6 1 3 2 2 1 8 4 吉非替尼+含培美曲塞的化疗 8 7 2 5 5 7 6 6 2 4 3 3 达可替尼 9 8 11 7 3 14 6 12 8 6 7 吉非替尼+培美曲塞 10 10 8 10 16 8 15 15 4 11 吉非替尼+阿帕替尼 11 11 14 11 15 13 11 11 7 6 11 10 阿法替尼+西妥昔单抗 12 13 3 9 14 7 7 7 12 阿法替尼 13 12 13 12 2 12 12 13 13 6 7 9 9 埃克替尼 14 16 10 14 6 15 14 14 厄洛替尼 15 9 15 13 4 8 9 10 10 13 8 吉非替尼 16 15 17 15 17 16 16 16 8 8 12 13 含培美曲塞的化疗 17 17 16 16 5 11 17 17 17 14 14 不含培美曲塞的化疗 18 18 18 17 18 18 18 18 15 15 表中红-黄-绿色阶反映各治疗方案SUCRA排名(红色代表更高排名,绿色代表更低排名)。 治疗方案 所有人群 突变 种族 吸烟 性别 脑转移 年龄 19Del L858R 亚洲 非亚洲 是 否 男性 女性 是 否 ≥65岁 <65岁 吉非替尼+含培美曲塞的化疗 1 4 3 1 7 5 1 7 1 1 3 6 阿法替尼+西妥昔单抗 2 1 5 5 2 3 4 1 5 达可替尼 3 5 2 2 3 10 1 5 2 4 3 厄洛替尼+雷莫芦单抗 4 吉非替尼+培美曲塞 5 3 埃克替尼+含培美曲塞的化疗 6 6 奥希替尼 7 3 10 12 1 8 3 4 3 2 2 2 4 奥希替尼+贝伐珠单抗 8 11 厄洛替尼+贝伐珠单抗 9 6 1 4 1 11 7 1 8 1 阿美替尼 10 5 阿法替尼 11 2 9 7 2 6 4 2 5 4 3 6 2 厄洛普尼 12 8 6 8 2 10 11 9 10 9 不啇鉤魎栉馀蓐哏峎陈培美曲塞的化疗 13 7 7 9 3 9 10 6 9 7 埃克替尼 14 9 8 13 4 7 6 11 伏美替尼 15 10 含培美曲塞的化疗 16 11 4 16 5 9 8 9 10 7 8 吉非替尼 17 10 11 14 4 11 6 8 8 3 4 5 10 吉非替尼+阿帕替尼 18 15 表中红-黄-绿色阶反映各治疗方案SUCRA排名(红色代表更高排名,绿色代表更低排名)。 在OS获益方面:①L858R突变/吸烟史/年龄<65岁/女性患者:厄洛替尼+贝伐珠单抗最优;②亚洲人群/男性/脑转移(无论有无):吉非替尼+培美曲塞化疗最优;②EGFR 19del/年龄≥65岁:阿法替尼+西妥昔单抗最优;③非亚洲人群:奥希替尼最优;④非吸烟患者:达可替尼最优。
在PFS获益方面:①亚洲人群/女性:厄洛替尼+贝伐珠单抗最优;②EGFR 19del:奥希替尼+贝伐珠单抗最优;③L858R突变/吸烟患者:埃克替尼+培美曲塞化疗最优;④非亚洲人群/年龄≥65岁:奥希替尼最优;⑤脑转移/非吸烟患者:阿美替尼最优;⑥男性患者:厄洛替尼+雷莫芦单抗最优;⑦无脑转移/年龄<65岁:伏美替尼最优。
-
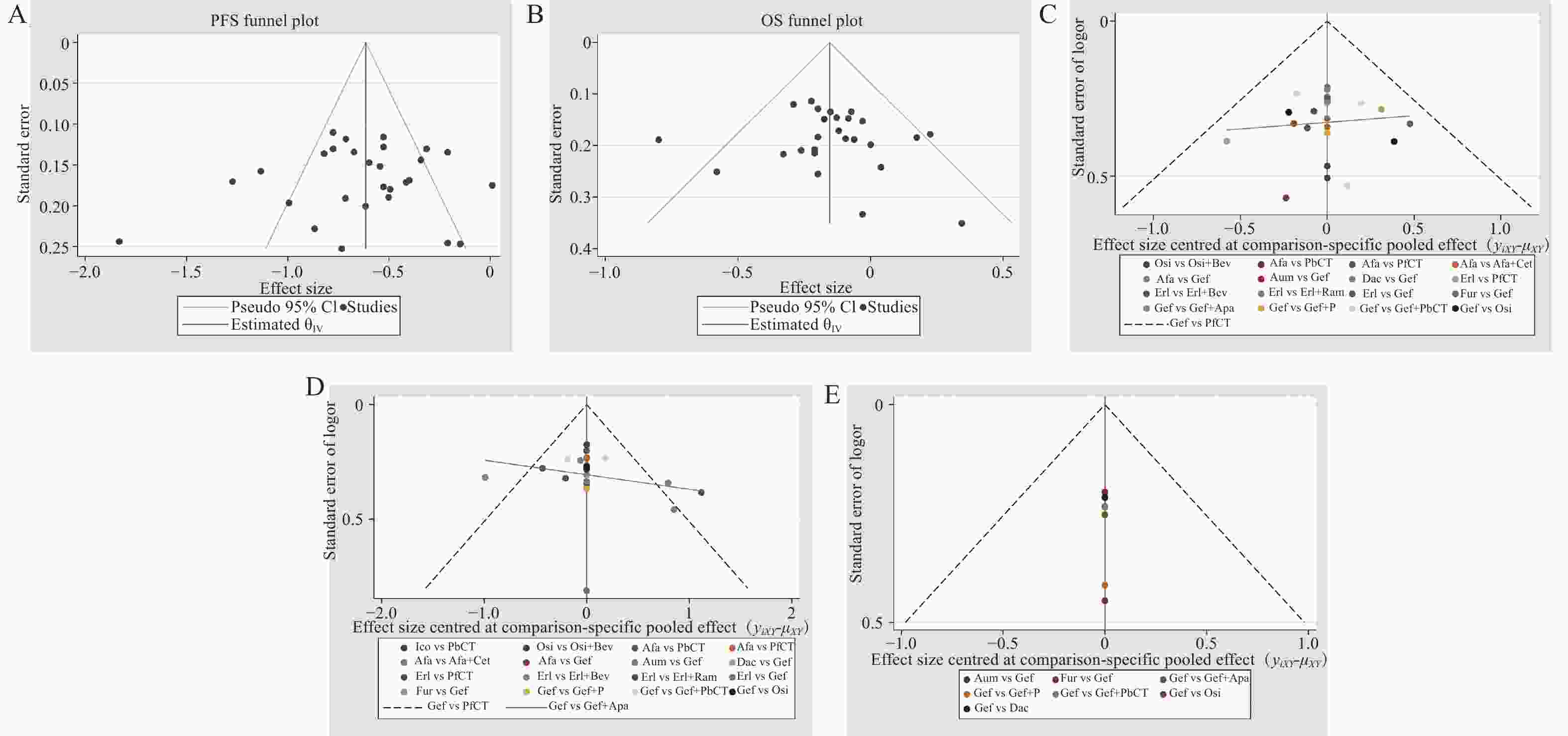
各结局指标的漏斗图见图6,分析显示,各研究散点对称分布,提示存在发表偏倚的可能性较低。此外,本研究通过排除Ⅱ期RCT,仅纳入Ⅲ期RCT进行敏感性分析,结果显示各结局指标排序结果较为稳健。
-
本研究通过网状Meta分析系统评估了18种EGFR-TKIs单药及联合方案的一线治疗价值。结果显示,吉非替尼联合含培美曲塞的化疗在OS和ORR方面具有显著优势,而奥希替尼联合贝伐珠单抗则在PFS方面更具优势。安全性方面,第三代EGFR-TKIs(如伏美替尼、奥希替尼)单药治疗的不良反应发生率最低,而联合治疗虽能提升疗效,但伴随更高的毒性风险。
就疗效而言,吉非替尼联合含培美曲塞化疗的OS优势可能与EGFR-TKIs和化疗药物之间的协同抗肿瘤作用相关。研究表明,T790M突变是第一、二代EGFR-TKIs获得性耐药的主要原因,而培美曲塞可能通过抑制T790M突变介导的耐药机制,从而增强吉非替尼的抗肿瘤效果 [53-54]。奥希替尼联合贝伐珠单抗与奥希替尼单药相比,显示出PFS获益的趋势,但差异并未达到统计学显著性(HR = 0.862,95%CrI:0.531~1.397) [50]。基于此,以奥希替尼为代表的第三代EGFR-TKIs仍被视为平衡疗效与安全性的优选,其优异表现主要与其不可逆结合EGFR突变体、穿透血脑屏障能力及对T790M的活性相关。
就安全性而言,联合治疗方案在提升疗效的同时,往往伴随着更为显著的毒性反应。以吉非替尼联合含培美曲塞的化疗方案为例,尽管其在OS和ORR方面均表现优异,位列所有治疗方案之首,但其AEs发生率也显著升高,这一结果凸显了联合治疗方案在疗效与安全性之间权衡的重要性。因此,在临床实践中,治疗方案的制定需要综合考虑患者的个体化特征,包括年龄、体能状态、合并症以及对不良反应的耐受能力。
本研究结果与Zhao等人[55]及Yang等人[56]的结论基本一致,均支持吉非替尼联合化疗的OS优势和奥希替尼的PFS及安全性优势。但本研究创新性在于:①纳入更多治疗方案(18种),覆盖最新三代TKIs(如伏美替尼、阿美替尼);②引入亚组分析,探索不同临床特征(如种族、脑转移)对疗效的影响,整合OS、PFS、ORR及安全性多维指标,提供更全面的方案排序。与此同时,本研究存在以下局限性:①部分RCT的OS数据成熟度不足,如伏美替尼OS数据成熟度仅29%,这可能导致结果的偏倚。②部分研究未明确疾病进展评估方式(研究者评估或第三方独立影像),可能对最终结果产生潜在影响。③SAEs网络证据图的星状结构(图4D)提示干预措施间缺乏直接比较数据,基于间接证据链的效应估计可能存在传递偏倚。
本研究为EGFR突变NSCLC一线治疗提供了循证依据,证实联合治疗在生存获益上的潜力,同时体现了第三代TKIs单药(如奥希替尼、伏美替尼、阿美替尼)在疗效-安全性平衡中的核心地位。此外,不同结局排序及亚组分析结果可为个体化治疗提供循证参考。随着临床研究数据的不断积累,未来仍需通过更长时间随访的RCT和真实世界研究进一步验证不同治疗方案间的疗效差异,并深入探索罕见靶点的精准治疗策略。
Efficacy and safety comparison of EGFR-TKIs monotherapy and combination therapy as first-line treatment for advanced non-small cell lung cancer: a network meta-analysis
doi: 10.12206/j.issn.2097-2024.202504044
- Received Date: 2025-04-08
- Accepted Date: 2025-07-23
- Rev Recd Date: 2025-06-20
- Available Online: 2025-11-17
-
Key words:
- EGFR-TKIs /
- NSCLC /
- EGFR mutation /
- network meta-analysis /
- first-line treatment
Abstract:
| Citation: | CHEN Guanxu, SONG Yutong, GUO Xiuqiang, ZHANG Mi, LIU Zhihong, SONG Hongtao. Efficacy and safety comparison of EGFR-TKIs monotherapy and combination therapy as first-line treatment for advanced non-small cell lung cancer: a network meta-analysis[J]. Journal of Pharmaceutical Practice and Service. doi: 10.12206/j.issn.2097-2024.202504044 |







 DownLoad:
DownLoad: