-
心力衰竭,简称心衰,是由于心脏器质性或功能性异常导致的心室充盈或射血能力受损所引起的复杂临床综合征[1]。随着人口老龄化程度不断加深以及许多患者从急性心脏疾病中存活,慢性心衰的患者数呈现增长态势,尽管过去30年其生存率有了显著提高,但5年病死率仍约为50%[2, 3]。慢性心衰因其高发病率和高病死率,长期以来一直是社会的主要医疗负担[4]。慢性心衰主要表现为射血分数降低的心衰,其药物治疗主要包括血管紧张素转换酶抑制剂或血管紧张素受体脑啡肽酶抑制剂、β受体阻滞剂、盐皮质激素受体拮抗剂、钠-葡萄糖协同转运体2抑制剂组成的四联疗法[5]。患者尤其是长期用药者,由于低血压、电解质紊乱等不良反应的影响,通常用药依从性欠佳。
传统中药在治疗心衰方面有着悠久的历史和独特的理论[6],具有多靶点且成本较低等优势,如黄芪[7]。黄芪注射液是临床上治疗心衰的常用中药注射剂之一,疗效肯定[8]。大量研究已经证实了黄芪治疗心衰的有效性和安全性[9, 10]。黄芪甲苷是黄芪治疗心衰的主要活性成分[11]。但是,由于黄芪甲苷水溶性差,生物利用度极低,限制了黄芪甲苷药物制剂在临床上的转化[12, 13]。因此,黄芪甲苷可作为先导化合物,进行结构优化,既保持其原有的活性,又能增强其水溶性,以便增加生物利用度,充分发挥黄芪甲苷治疗心衰的作用。本研究主要从合成的系列水溶性黄芪甲苷衍生物中,筛选具有抗心衰效用的化合物,并初步探讨其对心肌重塑和心肌纤维化的改善作用,以期为临床提供抗心衰的候选新药。
-
雄性C57BL/6小鼠150只,7~8周龄,体重为18~22 g,购自上海斯莱克实验动物有限公司,合格证编号:20170012004976。动物实验严格遵守动物护理伦理指南进行操作,已通过海军军医大学动物实验伦理委员会批准。饲养环境为无特定病原体(SPF)环境,温度为(25±2 )℃,每日光照12 h,昼夜循环,自由摄取标准饲料和饮用水。
-
水溶性黄芪甲苷结构改造衍生物包括HHQ、HHQ12CS和HHQ18TC,HHQ进一步结构改造得到HHQ16和HHQ19,均由上海医药工业研究院孙青䶮教授提供。卡托普利、羧甲基纤维素钠(CMC-Na)、异氟烷均购自国药集团。内源性过氧化物酶封闭液、DAB辣根过氧化物酶显色试剂盒、苏木素染色液购自碧云天公司。Masson复合染色试剂盒购自博谷德生物工程公司。Trizol试剂、逆转录试剂盒、SYBR Green Master Mix荧光定量试剂盒购自日本Takara公司。Target Retrieval Solution购自丹麦Dako公司。COL1、COL3抗体、TGF-β1抗体购自英国Abcam公司。αSMA抗体购自中国博奥森生物技术公司。
-
呼吸机(意大利UgoBasile公司);麻醉剂(中国惠德万邦公司);二维及M型超声(意大利EsaoteMyLab公司);诊断仪及探头(意大利One/Touch公司);光学显微镜(德国Leica公司);病理切片机(中国辅光精密仪器公司);包埋机(中国BIOBASE博科公司);组织摊片机(中国湖北孝感安立信公司);高通量组织研磨仪(中国上海必横生物公司);PCR扩增仪(德国Eppendorf公司);Stepone Plus实时荧光定量PCR仪(美国AB applied biosystems公司)。
-
通过2.5%异氟烷麻醉小鼠,气管插管接入呼吸机保持麻醉状态。开胸暴露心脏,在左心耳下缘约1 mm处用线缝扎冠状动脉,缝合后,待小鼠恢复自主呼吸,饲养3~4周,通过心超检测慢性心衰模型,从中筛选慢性心衰造模成功的小鼠(EF<50%)。
-
首先对黄芪甲苷衍生物进行药效筛选。将LAD结扎造模成功后的小鼠随机分为模型组、卡托普利组、系列黄芪甲苷衍生物(HHQ、HHQ12CS、HHQ18TC)组、假手术组,共6组。造模后4周使用0.5%CMC-Na溶液配制黄芪甲苷衍生物,并按照小鼠每10 g体重0.1 ml进行灌胃给药,黄芪甲苷衍生物组分别给予10 mg/kg的HHQ、HHQ12CS、HHQ18TC,假手术组和模型组分别给予同等剂量的0.5%CMC-Na溶液,卡托普利组给予8.6 mg/kg卡托普利混悬液,每日给药1次,连续28 d。HHQ衍生物药效筛选分组同上,即造模成功的小鼠随机分为模型组、HHQ组、HHQ16组和HHQ19组、假手术组,一共5组。假手术组和模型组处理同上,HHQ衍生物组分别用10 mg/kg的HHQ、HHQ16、HHQ19灌胃,每日给药1次,连续28 d。
-
通过吸入2.5%异氟烷气体麻醉小鼠,在检测过程中提供1.0%~1.5%浓度的异氟烷以维持麻醉状态。通过二维及M型超声诊断仪检测小鼠的心功能,筛选造模后的成功模型(EF<50%)以及评价给药后小鼠的心功能。主要检测指标有左室射血分数(LVEF)、左室短轴缩短率(LVFS)等参数。分别于给药18、28 d对各组小鼠进行心脏超声检测。
-
对小鼠进行心脏超声检测之后称重,然后取心脏称重。计算心脏重量和体重的比值,即心体比(mg/g)。心脏标本用10%福尔马林固定24 h后,将心脏取出,排列整齐,拍照,观察心脏的大小和形态。取小鼠心脏组织于4%多聚甲醛中固定24 h后,常规石蜡包埋切片处理。用HE染色和Masson染色观察心脏大体形态及胶原形成。
-
提取心肌组织总RNA,逆转录成cDNA,实时定量PCR体系包括:SYBR 10 μl,引物(上游引物和下游引物)1 μl,cDNA 3 μl,以及DEPC水7 μl,引物合成序列见表1。
名称 上游引物 下游引物 COL1A1 TAAGGGTCCCCAATGGTGAGA GGGTCCCTCGACTCCTACAT COL3A1 ACGTAGATGAATTGGGATGCAG GGGTTGGGGCAGTCTAGTG αSMA GGACGTACAACTGGTATTGTGC TCGGCAGTAGTCACGAAGGA TGF-β1 GAGCCCGAAGCGGACTACTA TGGTTTTCTCATAGATGGCGTTG Gapdh GTATGACTCCACTCACGGCAAA GGTCTCGCTCCTGGAAGATG -
心脏组织于4%多聚甲醛组织固定液中固定。梯度乙醇脱水后将组织浸入热石蜡中,将浸好的石蜡放入包埋机中包埋。将组织进行横切后去石蜡,使用Target Retrieval Solution进行抗原修复操作。用内源性过氧化物酶封闭液消除内源性过氧化物酶的干扰。用1%牛血清白蛋白封闭,使用相应的特异性抗体进行染色,根据种属关系选择对应二抗染色。为了可视化染色,使用DAB显色,并用苏木素进行复染。
-
实验处理及统计图表的绘制利用GraphPad Prism软件完成。实验结果均采用均数±标准误(mean ± SEM)的方式呈现。两组样本之间的比较采用两独立样本t检验(t-test);两组以上两两之间比较采用单因素方差分析(one-way ANOVA)或双因素重复方差分析(two-way ANOVA)。以P<0.05为差异有统计学意义。
-
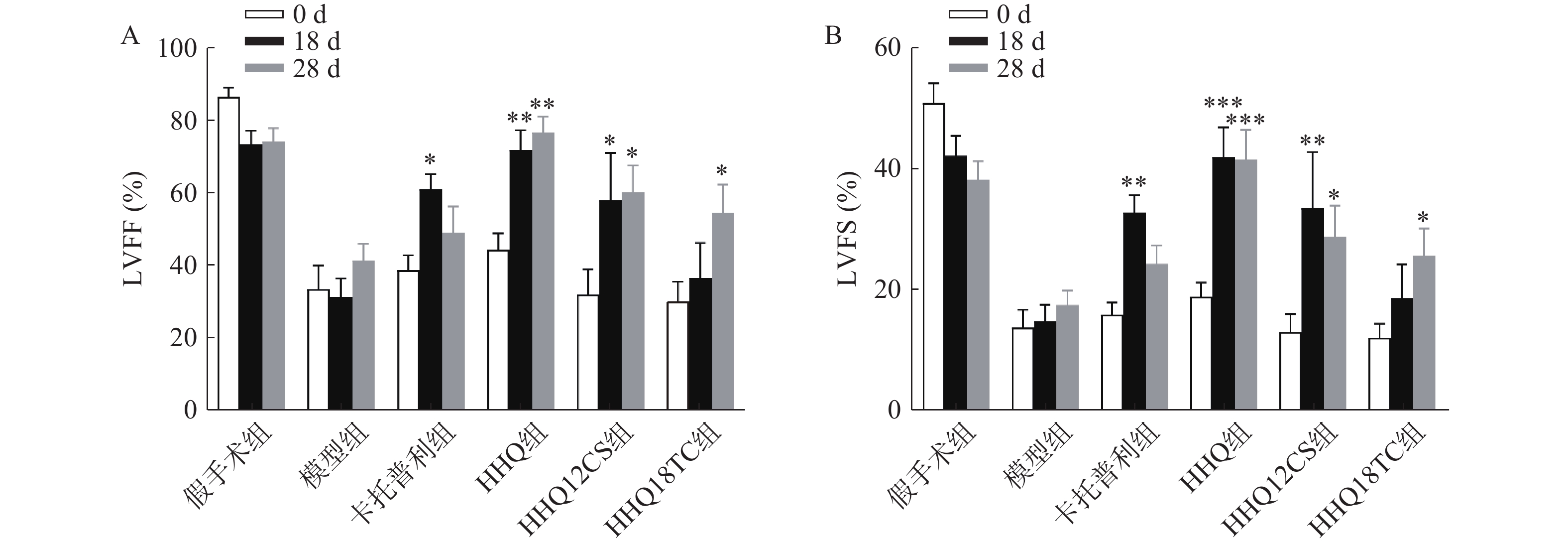
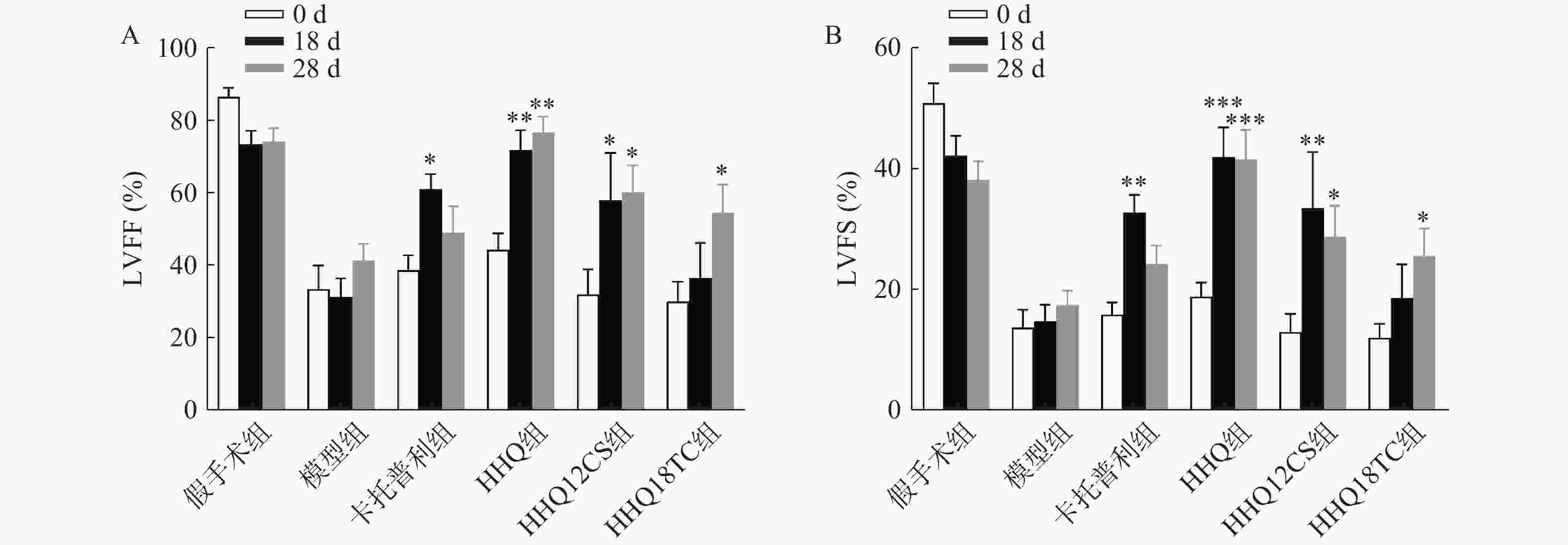
LVEF和LVFS是评价心衰的重要指标,心衰时LVEF和LVFS下降。如图1所示,阳性对照药卡托普利给药后增加18 d和28 d的LVEF和LVFS,以18 d最为显著,LVEF增长百分比为56%;黄芪甲苷衍生物HHQ、HHQ12CS、HHQ18TC均增加LVEF和LVFS,且呈时间依赖性,增强效果在3种药物之间无显著差异。以HHQ为例,LVEF增长百分比在18 d为61%,28 d为71%,优于同期的卡托普利。结合3种药物的合成难易性、稳定性、水溶性和药动学参数(数据在此不展示),HHQ可以作为进一步优化的候选化合物。
-
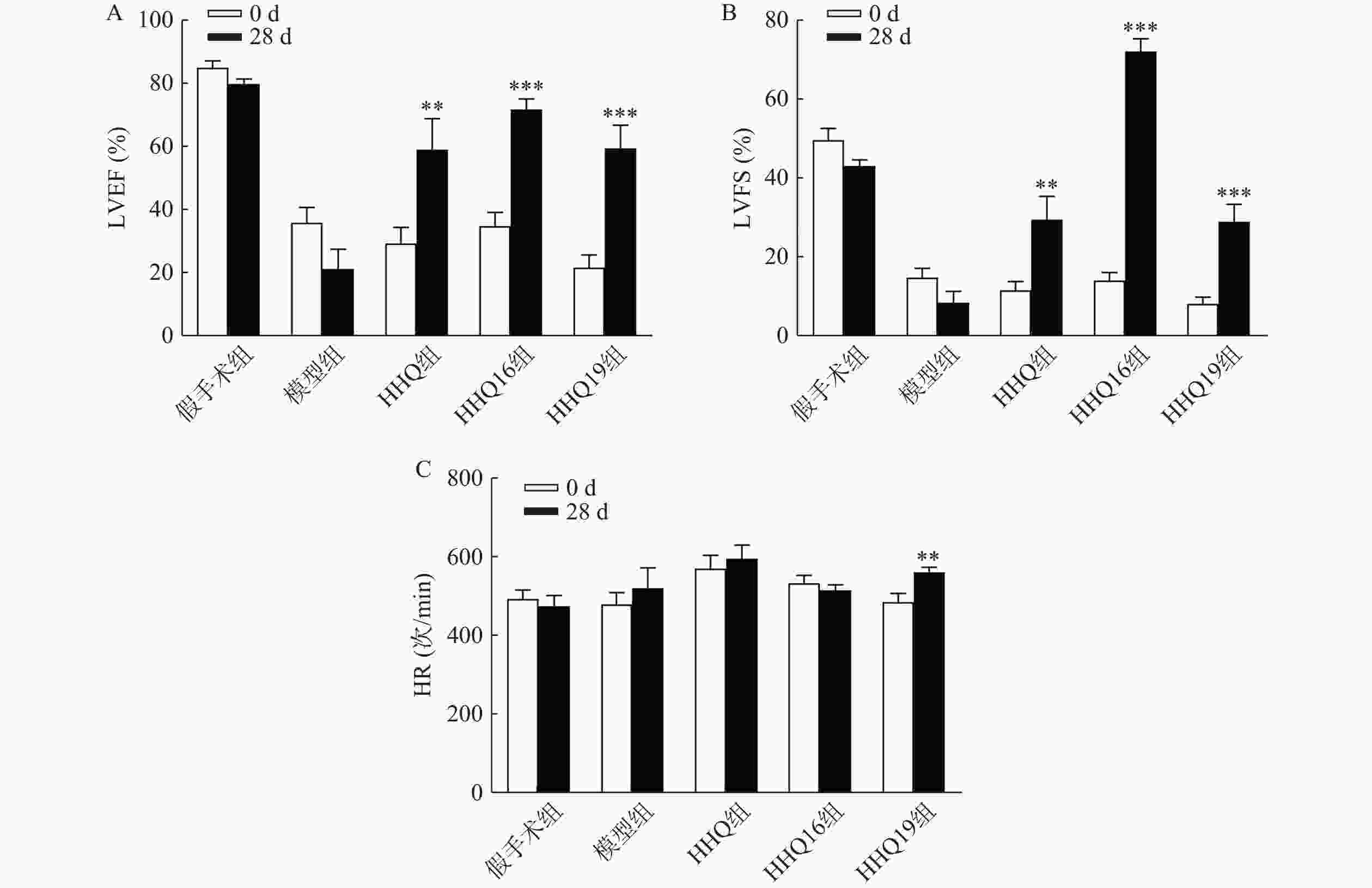
对上述筛选出来的HHQ药物进一步衍生化,使其具有更好的合成路线、稳定性、水溶性和药动学参数(数据不展示)。进一步以HHQ为对照,对其衍生物HHQ16和HHQ19进行药效评估。如图2所示,给药28 d,HHQ衍生物均能使得心衰小鼠的LVEF%和LVFS%升高,其中,HHQ16升高LVEF%的百分比为104%,HHQ19升高LVEF%的百分比为171%(图2A、图2B);而HHQ升高LVEF%的百分比为99%。但HHQ19显著增加心率(HR)(图2C),而HR增加会导致心肌耗氧量增加,增加死亡率[14]。因此,3种HHQ衍生物均能提升心衰小鼠的心功能,综合上述因素,选择HHQ16为最优候选化合物。
-
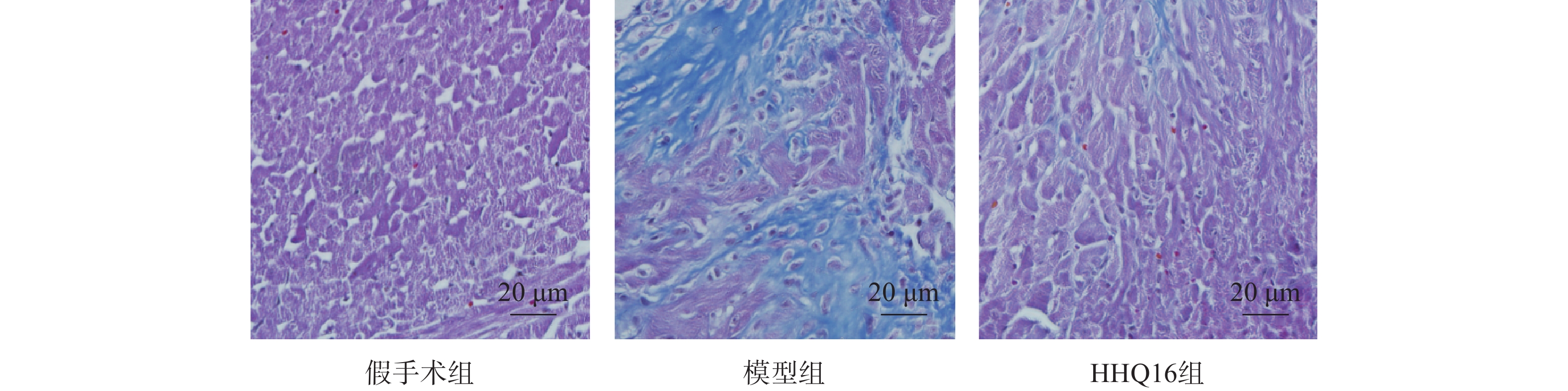
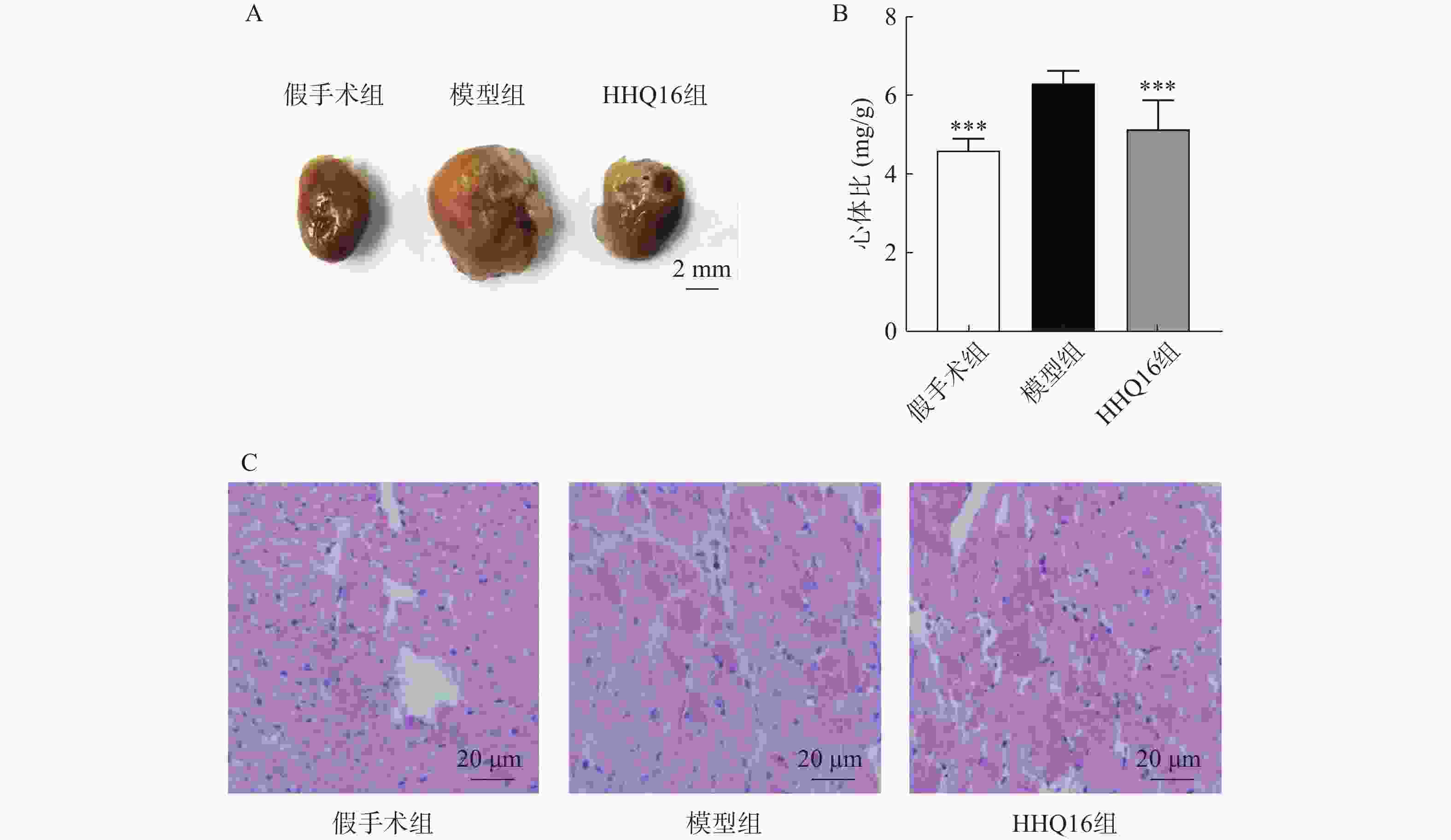
进一步观察候选化合物HHQ16对心肌重塑的影响,初步探索其作用机制。如图3所示,与假手术组比较,模型组心脏显著变大,形态不规则,心体比显著升高(图3B);HHQ16给药28 d后,心脏显著变小,心体比显著下降(图3A、图3B)。HE染色(图3C)表明,假手术组小鼠心肌细胞结构正常,模型组可见部分心肌细胞呈现区域性不规则,心肌细胞变大。HHQ16给药28 d后,形态变规则,心肌细胞肥大显著改善。
-
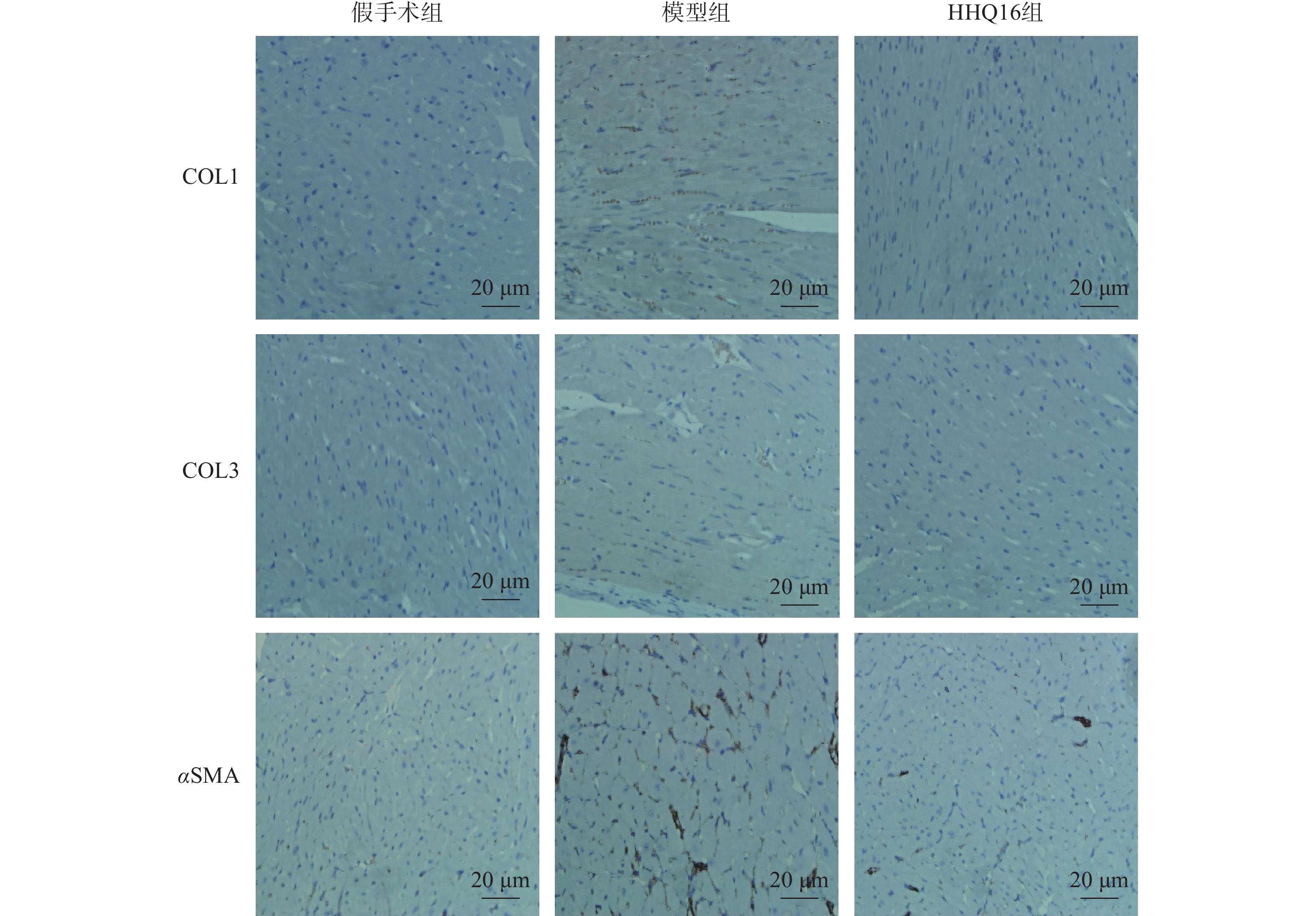
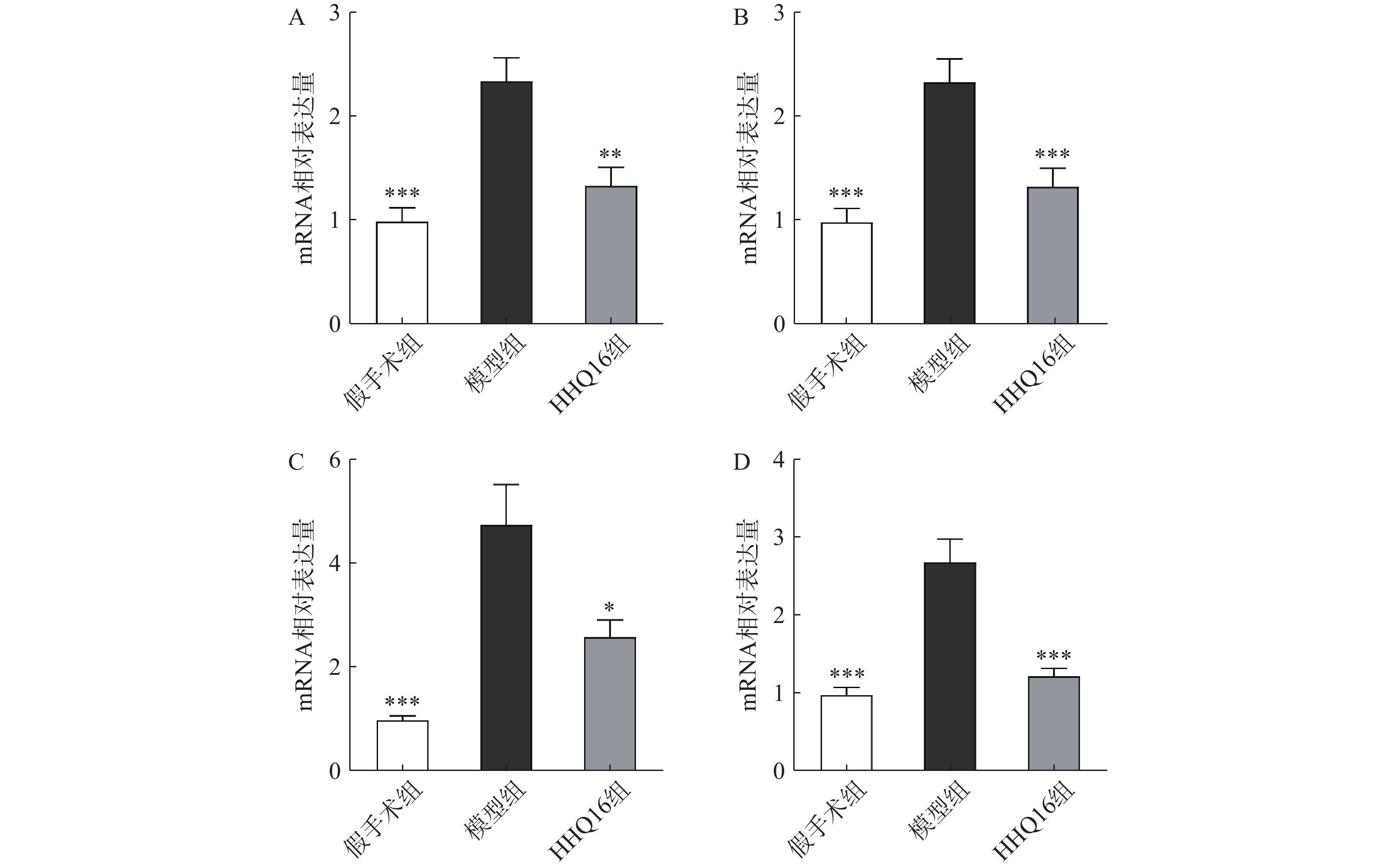
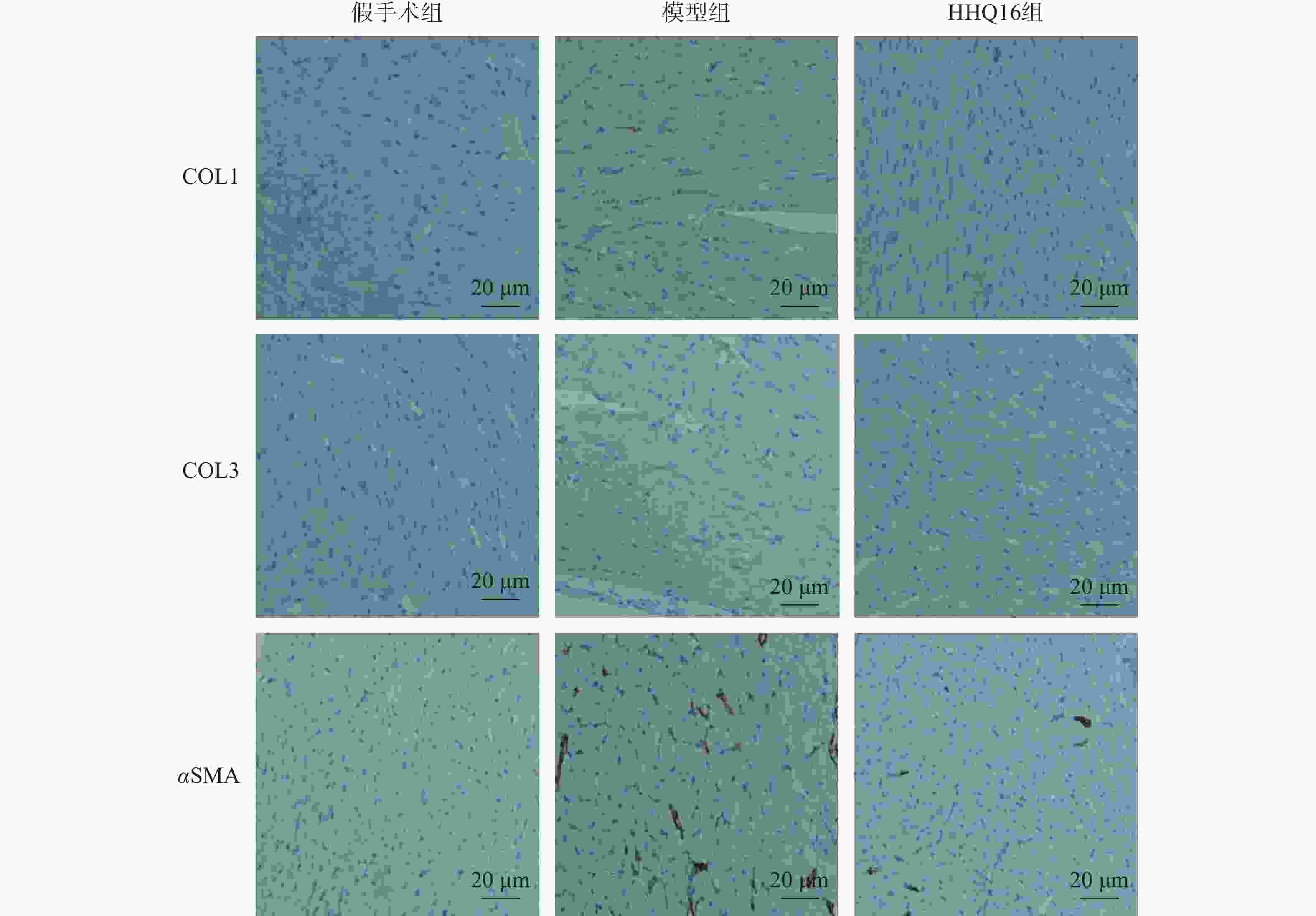
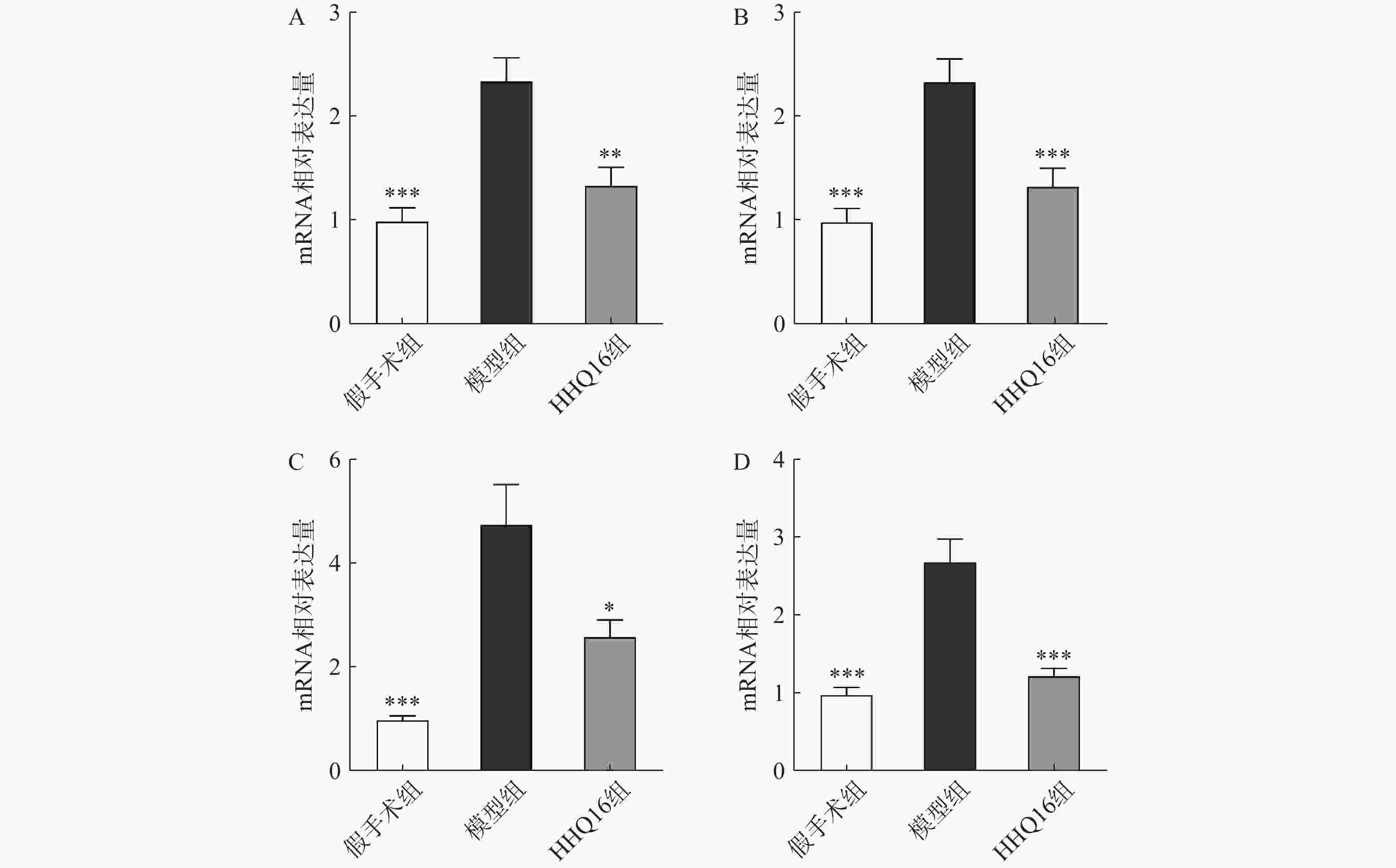
进一步观察HHQ16对心肌纤维化的影响。首先通过Masson染色,观察胶原沉积。如图4所示,假手术组的心肌组织无胶原沉积。模型组的心肌组织胶原沉积增多;而HHQ16组给药28 d,心肌胶原沉积显著减少。COL1、COL3、αSMA和TGF-β1是评估心肌纤维化的重要指标[15]。免疫组织化学染色结果显示(图5),与假手术组比较,模型组COL1、COL3、αSMA蛋白表达水平显著升高,经HHQ治疗后,这些蛋白表达水平显著降低。基因水平也展示了相似的改变(图6),与假手术组比较,模型组COL1、COL3、αSMA和TGF-β1 mRNA表达水平显著升高,而HHQ16能显著降低这些纤维化指标的mRNA表达水平。以上结果表明,HHQ16可以改善心衰造成的心肌纤维化。
-
心衰已经成为严重威胁人类健康的全球性公共卫生问题。越来越多的研究表明,由于“多靶点效应”,中药治疗心衰具有独特的优势,中药活性成分为新药开发提供了有价值的候选化合物。黄芪甲苷是治疗慢性心衰的中成药黄芪注射液的主要有效成分。然而,黄芪甲苷结构复杂,水溶性差,生物利用度低,化学合成困难,限制了该药物的临床转化。本研究涉及的水溶性黄芪甲苷衍生物均可显著改善心功能,最终根据化合物合成难易、药物稳定性、水溶性以及初步药动学比较,确定HHHQ16为最优候选药物。HHQ16具有抗心衰作用,对左冠状动脉前降支结扎模型诱导的慢性心衰小鼠有明确的治疗效果。
心肌重塑是由一系列复杂的分子和细胞机制导致的心肌结构、功能和表型的变化,包括心肌细胞肥大及心肌纤维化(心肌基质重构)等改变,以及在此基础上形成的心脏扩大、心脏质量增加。目前的研究认为,心肌重塑是心衰发生、发展的分子细胞学基础,是心衰恶化的主要机制之一。相关研究表明黄芪甲苷具有抗氧化、增强免疫力、增强心肌收缩力、减轻心肌纤维化和心肌重塑等作用[16-20]。通过对心衰小鼠表型观察,发现经过LAD手术后,模型组的心脏显著变大,心肌细胞变大,结构不规则,通过HHQ16治疗28 d后,心脏显著变小,心肌细胞接近正常,形态变规则。表明HHQ16能够改善心衰小鼠心肌重塑。
心肌纤维化被认为是心衰的基本病理变化,也是心肌重塑的分子基础[21]。心肌纤维化是当心肌因缺血、炎性反应、衰老等原因受损时,局部的心肌细胞凋亡,心肌成纤维细胞增殖,胶原纤维为主的细胞外基质增多,从而产生的一种病理变化。其主要特征是胶原蛋白合成和降解之间的平衡紊乱,导致心脏的深层结构和功能异常,造成收缩和舒张功能的严重损害,最终体现为心衰[22, 23]。心肌纤维化与心衰患者的疾病严重程度密切相关,但纤维化过程尚未成为心衰的直接治疗靶标。实验和临床证据均表明,心肌纤维化的改变可能是可逆的[22],开发预防和逆转心肌纤维化的药物对于减轻心肌重塑和延缓心衰的发展至关重要。
胶原蛋白是细胞外基质的重要组分,其中COL1约占心脏胶原总量的85%,参与组成具有抗张强度的粗纤维,COL3约占11%,可组装成细纤维,以保持基质网的弹性[24]。在以心肌纤维化为特征的心脏疾病中,不管其病因如何,TGF-β1通常是导致纤维化的最后的、共同的中介物之一。TGF-β1是最重要的促纤维化生长因子之一,通过调节前胶原蛋白基因的表达,促进合成细胞外基质,促使心肌纤维化[25]。在肌成纤维细胞中,αSMA被组装成应力纤维,进一步导致细胞外基质蛋白过度沉积,减弱组织顺应性并加速心衰,肌成纤维细胞的分化是心肌纤维化反应的标志,其中,α-SMA的表达是此种分化的重要特征[26]。在本研究中,用Masson染色检测了小鼠的胶原,LAD手术可使小鼠胶原明显增多,而HHQ16给药治疗后能有效减少胶原沉积。通过免疫组化染色和qPCR检测了小鼠心肌纤维化相关指标,发现HHQ16可下调COL1、COL3以及αSMA蛋白水平的表达,并且下调COL1、COL3、αSMA和TGF-β1 mRNA水平的表达,从而改善LAD手术引起的心肌纤维化,改善心衰程度。
综上所述,本研究从黄芪甲苷衍生物对心衰小鼠的保护作用入手,筛选出改造后黄芪甲苷衍生物最优的候选化合物HHQ16,通过对心肌重塑和心肌纤维化相关指标的检测,初步研究了其在心衰治疗中的作用机制,为HHQ16治疗心衰的药物研发提供科学依据。
Efficacy and mechanism of astragaloside Ⅳ derivatives on chronic heart failure in mice
doi: 10.12206/j.issn.2097-2024.202310004
- Received Date: 2023-10-03
- Rev Recd Date: 2024-02-18
- Available Online: 2024-05-22
- Publish Date: 2024-05-25
-
Key words:
- astragaloside Ⅳ derivative /
- chronic heart failure /
- cardiac function /
- myocardial fibrosis
Abstract:
| Citation: | JING Kai, YANG Cirong, ZHANG Zhen, ZANG Yibei, LIU Xia. Efficacy and mechanism of astragaloside Ⅳ derivatives on chronic heart failure in mice[J]. Journal of Pharmaceutical Practice and Service, 2024, 42(5): 190-197. doi: 10.12206/j.issn.2097-2024.202310004 |








 DownLoad:
DownLoad: