-
钙调神经磷酸酶抑制剂(CNI)于20世纪90年代引入器官移植领域,是大多数实体器官移植免疫抑制方案的基石[1-2]。目前,94%的肾移植受者在移植后使用基于CNI的免疫抑制方案出院,90%以上的受者接受他克莫司治疗[3-4]。他克莫司主要通过细胞色素P450(如CYP3A4和CYP3A5等)代谢,由P-糖蛋白(P-gp)进行转运。研究表明,CYP3A5酶是他克莫司在人体主要的代谢酶,MDR1(编码P-gp的基因)的基因多态性也是肾移植术后他克莫司血药浓度的影响因素[5-6]。
《器官移植免疫抑制剂临床应用技术》(2019版)建议他克莫司服药后一个月内血药浓度需控制在8~12 ng/ml[7],各移植中心的靶浓度根据具体给药方案、血药浓度检测试剂盒的规定而确定。我院器官移植中心靶浓度为10~15 ng/ml。但由于通常器官移植术后 5~7 d他克莫司的药物浓度达到稳态,这使得他克莫司首次给药剂量与首次的血药浓度检测值可能存在一定的时间差,因此需要尽早对给药剂量进行干预,以达到提高他克莫司的疗效和减少其不良反应的目的。
由于肾功能通常不会在肾移植术后完全恢复,移植受者具有慢性肾脏病第2或第3期的特征[8],因此,本研究对我院在2013年至2017年做肾移植手术的尿毒症患者的CYP3A5(CYP3A5*3,6986A>G)及MDR1(C3435>T,G2677 >T/A,C1236>T)基因型进行检测,探讨其对于他克莫司血药浓度、血药浓度/剂量比值的影响,以及与最佳治疗浓度范围和患者肌酐水平的相关性,为开展早期肾移植患者的免疫抑制剂个体化管理,避免浓度过低造成免疫不足或浓度过高产生不良反应提供依据。
-
选取2013年7月至2017年7月在上海长征医院被诊断为尿毒症,并接受同种异体肾移植的131例受者。其中男性患者89例,女性患者42例,年龄区间在6~68岁,无其他严重疾病。所有被选取的研究对象均签署了知情同意书。
入选标准:①首次进行肾移植手术的患者;②在治疗过程中使用他克莫司作为免疫抑制剂;③符合诊断标准,无其他严重疾病。排除标准:排除术前术后使用CYP3A酶诱导剂或抑制剂。
用药方案:临床诊断患者为慢性肾衰竭尿毒症期,后接受肾移植手术,术后采用他克莫司+霉酚酸类药物+激素的三联方案作为免疫抑制治疗方案。他克莫司初始剂量为0.1 mg/(kg·d),分2次给药。
-
患者于入院检查时抽取外周血2 ml(EDTA-2K抗凝管),焦磷酸测序方法检测CYP3A5(CYP3A5*3,6986A>G)及MDR1(C3435>T,G2677>T/A,C1236>T)的基因型。
-
患者前1晚8时服用他克莫司,次日早晨8时服药前抽取外周血2 ml(EDTA-2K抗凝管),通过微粒子酶免疫分析(美国雅培公司ARCHITECT i1000SR)检测他克莫司浓度。
-
收集整理以上患者的他克莫司血药浓度、给药剂量、肌酐水平、红细胞比容,使用统计学方法比较患者基因型对于血药浓度的影响,研究免疫抑制剂最佳浓度范围。
-
使用SPSS20.0软件进行统计学分析,计量资料均以(
$\bar x $ ±s)表示,采用χ2检验考察肾移植受者基因型上的等位基因分布是否符合Hardy-Weinberg平衡定律,采用非参数检验Mann-Whitney U检验比较2组组间是否存在显著性差异,使用Kruskal-Wallis检验多个独立样本之间是否存在显著性差异,P≤0.05具有统计学意义。 -
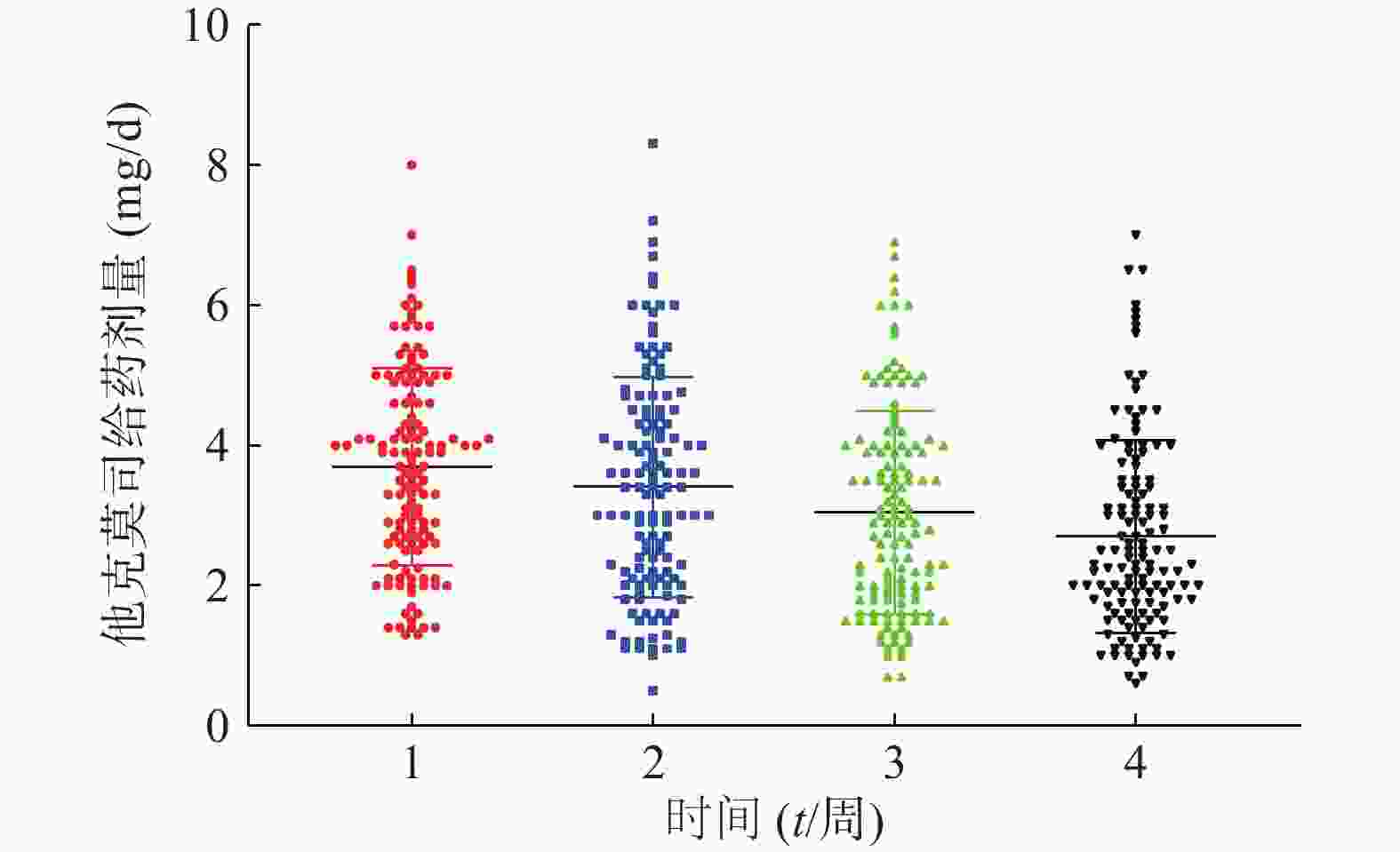
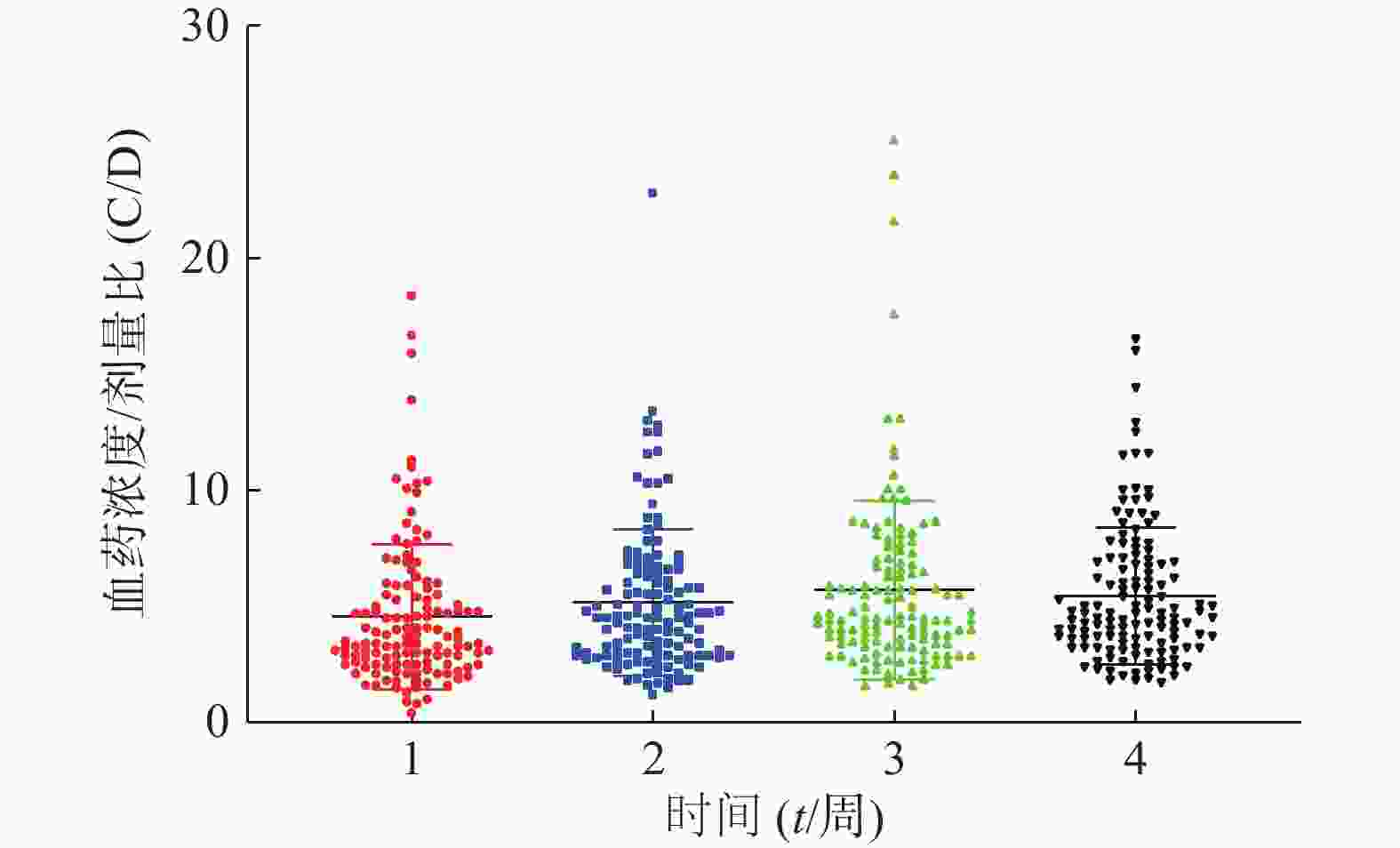
患者在术后3周内的平均血药浓度趋近于14 ng/ml,如图1。患者术后每周他克莫司的平均给药剂量均在逐步减少,分别为(3.7±1.4)、(3.4±1.6)、(3.0±1.5)、(2.7±1.4)mg/d,如图2,但是不同患者的给药剂量差异较大,每周最大差异分别达到6.15倍、16.6倍、9.86倍和11.67倍。患者在术后1个月内的血药浓度与给药剂量的比值存在一个逐渐增大的过程,如图3,在第3周左右出现最大值。
-
CYP3A5*3(A6986G)常见基因型为AA、AG、GG;MDR1(C1236T)常见基因型为CC、CT、TT;MDR1(G2677T/A)常见基因型为GG、GA、AA、GT、TT、AT;MDR1(C3435T)常见基因型为CC、CT、TT。在131例接受肾移植手术的尿毒症患者中,通过焦磷酸测序法测得患者CYP3A5和MDR1基因型的分布情况,详情见表1。经过χ2检验,结果显示以上各基因型的分布均符合Hardy-Weinberg平衡定律(P>0.05)。
基因 基因型 例数 百分比(%) CYP3A5*3
(A6986G)AA 7 5.3 AG 56 42.7 GG 68 51.9 MDR1
(C1236T)CC 49 37.4 CT 63 48.1 TT 19 14.5 MDR1
(G2677T/A)GG 29 22.1 GA 20 15.3 AA 1 0.8 GT 46 35.1 TT 17 13 AT 18 13.7 MDR1
(C3435T)CC 54 41.2 CT 58 44.3 TT 19 14.5 -
基因型 时间
(周)血药浓度
(ng/ml)给药剂量
(m/mg)浓度/剂量比
(C/D)CYP3A5*1/*1
(AA)1 13.3±6.0 4.4±2.1 3.6±3.0 2 12.4±3.9 4.7±2.3 3.0±1.3 3 12.6±4.7 4.5±2.0 3.0±0.9 4 11.4±3.4 4.4±1.9 2.9±1.2 CYP3A5*1/*3
(AG)1 13.6±5.4 4.3±1.3 3.5±2 2 14.7±5.4 4.1±1.4 4.0±2.0 3 14.3±4.4 3.6±1.3 4.4±1.7 4 12.0±3.3 3.1±1.3 4.3±1.6 CYP3A5*3/*3
(GG)1 14.9±6.6 3.1±1.2 5.6±3.7 2 14.4±5.1 2.7±1.3 6.4±3.6 3 13.7±4.4 2.4±1.2 7.1±4.7 4 11.9±3.5 2.2±1.2 6.7±3.3 术后1~2周内基因型为CYP3A5*1/*1(AA)和基因型为CYP3A5*1/*3(AG)的肾移植受者在血药浓度、给药剂量和浓度剂量比方面无显著性差异(P>0.05)。第3周和第4周剂量浓度比值方面存在显著性差异(P<0.05),基因型为*1/*1(AA)的浓度剂量比较小,其他各组在血药浓度和给药剂量方面无显著性差异(P>0.05)。
术后1~4周内基因型为CYP3A5*1/*1(AA)和基因型为CYP3A5*3/*3(GG)的肾移植受者在血药浓度方面无显著性差异(P>0.05),第1周内基因型为*1/*1(AA)与*3/*3(GG)的患者在给药剂量和浓度剂量比方面无显著性差异(P>0.05),但前者的平均给药剂量大于后者。在第2周到第4周*1/*1(AA)和*3/*3(GG)的患者在给药剂量和浓度剂量比值存在显著性差异(P<0.05)。
术后1~4周内基因型为CYP3A5*1/*3(AG)和基因型为CYP3A5*3/*3(GG)的肾移植受者在血药浓度方面无显著性差异(P>0.05),在给药剂量和浓度剂量比方面具有显著性差异(P<0.05)。
-
术后1~4周 MDR1(C1236T)(表3),MDR1(G2677T/A)(表4)以及MDR1(C3435T)(表5)各基因的基因型患者,仅在MDR1(G2677T/A)基因型的患者术后第2周的血药浓度方面存在显著性差异(P<0.05),其余各基因的基因型患者血药浓度,给药剂量和浓度/剂量比方面均无显著性(P>0.05)。
基因型 时间
(周)血药浓度
(ng/ml)给药剂量
(m/mg)浓度/剂量比
(C/D)MDR1(C1236T)
CC1 12.6±4.6 3.7±1.4 4.1±2.5 2 15.4±5.3 3.4±1.7 5.2±1.9 3 13.8±4.0 3.0±1.6 6.3±5.2 4 12.0±2.6 2.8±1.7 5.7±3.4 MDR1(C1236T)
CT1 15.1±6.8 3.7±1.4 4.6±2.9 2 13.6±4.9 3.5±1.6 5.0±3.5 3 13.7±5.1 3.2±1.5 5.2±3.4 4 11.8±3.4 2.9±1.4 5.1±2.8 MDR1(C1236T)
TT1 13.7±5.5 3.7±1.4 4.6±3.7 2 15.0±5.4 3.4±1.6 5.3±3.1 3 14.1±3.4 3.0±1.4 6.0±3.8 4 12.0±3.7 2.6±1.3 5.8±3.0 基因型 时间
(周)血药浓度
(ng/ml)给药剂量
(m/mg)浓度/剂量比
(C/D)MDR1(G2677T/A)
AA1 12.5±0 3.9±0 3.2±0 2 18.5±0 3.6±0 5.1±0 3 9.9±0 3.4±0 2.9±0 4 12.6±0 3.3±0 3.8±0 MDR1(G2677T/A)
AT1 15.8±6.2 3.8±1.5 5.0±3.7 2 13.1±4.8 3.5±1.6 5.3±5.0 3 12.6±4.1 3.4±1.6 4.3±1.9 4 12.2±3.6 3.4±1.7 4.5±2.3 MDR1(G2677T/A)
GA1 13.5±6.6 3.4±1.2 4.5±2.8 2 12.0±5.0 3.1±1.3 4.3±1.8 3 13.2±4.6 2.9±1.3 5.4±2.8 4 11.7±3.0 2.7±1.3 5.7±3.5 MDR1(G2677T/A)
GG1 14.1±6.0 3.9±1.6 4.1±2.2 2 16.3±5.9 3.5±1.8 5.8±3.4 3 15.9±5.0 3.0±1.8 7.6±6.1 4 11.2±3.0 2.6±1.6 5.7±3.2 MDR1(G2677T/A)
GT1 14.7±6.6 3.7±1.5 4.8±3.5 2 14.6±4.6 3.5±1.6 5.1±2.5 3 13.6±4.3 3.0±1.4 5.5±2.9 4 11.7±3.7 2.5±1.1 5.5±2.8 MDR1(G2677T/A)
TT1 12.8±4.0 3.5±1.1 4.4±3.4 2 14.5±4.9 3.4±1.3 5.3±3.2 3 13.7±2.3 3.1±1.2 5.2±2.5 4 13.5±3.4 2.7±1.2 6.0±3.0 基因型 时间
(周)血药浓度
(ng/ml)给药剂量
(m/mg)浓度/剂量比
(C/D)MDR1(C3435T)
CC1 14.7±6.6 3.8±1.4 4.4±2.5 2 14.8±5.5 3.4±1.6 5.3±3.0 3 14.7±4.9 3.0±1.5 6.7±5.2 4 11.8±3.0 2.6±1.4 5.8±3.4 MDR1(C3435T)
CT1 14.6±5.9 3.7±1.5 4.8±3.6 2 14.1±4.8 3.5±1.6 5.1±3.3 3 13.4±4.2 3.1±1.5 5.0±2.2 4 11.7±3.7 2.8±1.4 5.0±2.4 MDR1(C3435T)
TT1 11.9±4.8 3.4±1.1 4.1±3.4 2 14.4±5.5 3.3±1.3 5.2±3.2 3 13.2±2.7 2.9±1.2 5.2±2.5 4 13.0±3.6 2.6±1.2 5.9±2.9 -
以患者术后1个月的血药浓度为依据,将患者分为5组,由表6可知:各组的肌酐水平存在显著性差异(P<0.05),而各组患者红细胞比容、年龄及性别则无显著性差异(P>0.05)。可以看出他克莫司血药浓度控制在10~13 ng/ml时患者的肌酐水平最接近于正常值。
项目 血药浓度
(ng/ml)V>16血药浓度(ng/ml)
16>VI≥13血药浓度(ng/ml)
13>III≥10血药浓度(ng/ml)
10>II≥7血药浓度(ng/ml)
17>I≥4性别 16 (M); 6 (F) 10 (M);10 (F) 35 (M);12 (F) 22 (M);8 (F) 2 (M);2 (F) 年龄(y) 42.5±13.9 39.2±15.2 41.7±13.2 40.9±14.8 32.3±6.8 红细胞比容(%) 31.9±4.7 31.3±4.3 34.5±12.1 34.2±9.9 28.7±8.5 肌酐(μmol/L) 216.7±196.8 157.6±123.3 128.8±67.3 159.5±266.4 174.8±47.5 -
在《中国肾移植后免疫抑制治疗指南》中,他克莫司+霉酚酸类药物+激素类药物的三联疗法已经成为临床上治疗肾移植后免疫排斥反应的默认疗法。指南中规定,以肾移植受者服用他克莫司后次日晨服药前的谷浓度作为检测指标,在接受手术后1个月内血药浓度要控制在10~15 ng/ml,3个月以内为8~15 ng/ml,一年以内为5-12 ng/ml [1]。本研究发现肾移植术后1个月时,为了维持目标血药浓度,不同受者之间他克莫司所需要的剂量差异最高可高达16.6倍,此情况与前期文献报道的最大差异可高达12倍的情况一致[9]。
研究发现,CYP3A5*3的基因型突变出现在中国人群中的概率高达78.8%[10],因此, CYP3A5基因型检测已经成为目前器官移植患者使用他克莫司个体化给药的必备手段。本研究的结果表明,术后1~4周基因型为AG的患者在给药剂量及浓度剂量比方面与基因型为GG的患者存在显著性差异(P<0.05),术后2~4周基因型为AA的患者在给药剂量及浓度剂量比方面与基因型为GG的患者存在显著性差异(P<0.05)。CYP3A5*3等位基因的存在对他克莫司血药浓度具有显著性影响。可以看出不同基因型的患者在他克莫司血药浓度维持在治疗浓度的时候,带有CYP3A5*3等位基因的患者给药剂量小于带有CYP3A5*1等位基因的患者。浓度/剂量比也反映了CYP3A5等位基因的出现会导致他克莫司代谢的降低,而且CYP3A5*3等位基因的数量越多,对代谢的影响就越大。即对他克莫司的代谢速率,纯合子>杂合子>野生型,野生型的基因型代谢速率可能近似于杂合子和纯合子的50%~70%。因此,在临床上对这类患者需要降低他克莫司的给药剂量,以避免出现毒副作用。
对于不同MDR1基因型的患者,仅发现患者为MDR1(G2677T/A)基因的情况下,术后第2周他克莫司血药浓度具有显著性差异(P<0.05),其余均无显著性差异。在131例肾移植受者中,仅有1例MDR1(G2677T/A)AA的患者,可能导致分析的不准确。现有关于MDR1基因多态性对他克莫司血药浓度的影响意见也各不相同。Meta分析显示亚洲人群肾移植患者术后他克莫司血药浓度/剂量值与MDR1基因C3435T多态性相关, 其相关性为TT>CT>CC。有报道认为MDR1基因G2677T/A突变型的患者中, 他克莫司的血药浓度比野生型个体高44.7% [11]。本研究表明,CYP3A5(*1*1)每日对他克莫司剂量需求最高,CYP3A5(*3*3)最低,与文献报道一致[12]。以往的研究表明,当CYP3A5基因型出现突变时,MDR1基因对他克莫司血药浓度的影响很小[13-14]。如果将研究局限于CYP3A5基因型为AA的患者中,则携带4~6个MDR1等位基因的患者较携带0~3个等位基因的患者具有较高的剂量要求[15]。由于本研究中CYP3A5基因型为AA的患者仅有7例,受限于样本量过小,难以说明MDR1基因型对于他克莫司血药浓度的影响。
临床实践和众多的文献表明,遗传因素是造成肾移植受者他克莫司谷浓度及浓度/剂量比个体差异的重要原因,我们的研究结果也与之一致。更重要的是,我们发现同一基因型的患者,他克莫司的浓度/剂量比在早期呈现逐渐减小的趋势,与之对应的给药剂量随着移植时间延长逐渐减小,且肌酐逐渐降低接近至正常人水平,说明患者肾功能处于恢复期。当患者血药浓度为10~13 ng/ml的时候,患者移植的肾功能最接近正常人肾功能水平。因此,采用现代分子生物学技术在移植术前检测患者的基因型,这对依据其基因型确定器官移植术后免疫抑制剂的剂量、改善患者预后有重要的指导意义。
Effect of CYP3A5 and MDR1 gene polymorphism on blood concentration of tacrolimus and creatinine level in uremic patients during the early phase of kidney transplantation
doi: 10.12206/j.issn.1006-0111.202009087
- Received Date: 2020-09-26
- Rev Recd Date: 2022-01-04
- Available Online: 2022-03-29
- Publish Date: 2022-03-25
-
Key words:
- tacrolimus /
- kidney transplantation /
- creatinine /
- CYP3A5 /
- MDR1
Abstract:
| Citation: | WEN Yan, ZHU Dekang, FU Shangxi, DENGYi, ZHANG Feng, CHEN Wansheng. Effect of CYP3A5 and MDR1 gene polymorphism on blood concentration of tacrolimus and creatinine level in uremic patients during the early phase of kidney transplantation[J]. Journal of Pharmaceutical Practice and Service, 2022, 40(2): 165-170. doi: 10.12206/j.issn.1006-0111.202009087 |










 DownLoad:
DownLoad:

