-
市售复方酮康唑乳膏包含酮康唑、硫酸新霉素和丙酸氯倍他索,是治疗浅部真菌感染的常用药物。其中,酮康唑是最常用的抗真菌药物,具有价格低、抗菌谱广、抗真菌活性强等优势。但细菌对硫酸新霉素易产生耐药性,可导致患者反复感染,难以根治,且丙酸氯倍他索不适合12岁以下儿童使用,副作用较多,可产生红斑、灼热、瘙痒等刺激症状,长期大面积用药可导致高血糖等[1-5]。针对上述问题,为改善市售产品的有效性和安全性,本课题组将硫酸新霉素替换为抗菌作用更强的莫匹罗星,将丙酸氯倍他索替换为副作用较少的糠酸莫米松,再结合酮康唑,制备新型复方酮康唑软膏,以提高患者的用药依从性[6-8]。
本研究采用反相高效液相色谱法同时测定复方酮康唑软膏中酮康唑、莫匹罗星和糠酸莫米松3种药效成分的含量,该方法目前未见有文献报道。本法简便,灵敏,分离度好,准确性高,可以为该制剂的质量标准研究提供依据。
HTML
-
AL204型电子天平(梅特勒-托利多仪器有限公司);TU-1901型紫外可见分光光度计(北京普析通用仪器有限责任公司);Agilent 1200型高效液相色谱仪(美国Agilent公司);Starter 2C型实验室pH计(奥豪斯仪器有限公司);KQ-800KDE型超声波清洗器(昆山市超声仪器有限公司)。
-
酮康唑对照品(批号:100294-201203,含量99.4%)、莫匹罗星对照品(批号:130568-200501,含量94.2%)、糠酸莫米松对照品(批号:100930-201201,含量99.9%)均购自中国食品药品检定研究院;酮康唑原料药(批号:20130405)、莫匹罗星原料药(批号:20130301)、糠酸莫米松原料药(批号:20130228)均购自武汉鑫佳公司;聚乙二醇400和聚乙二醇3350(中国医药对外贸易公司)。
1.1. 仪器
1.2. 药品与试剂
-
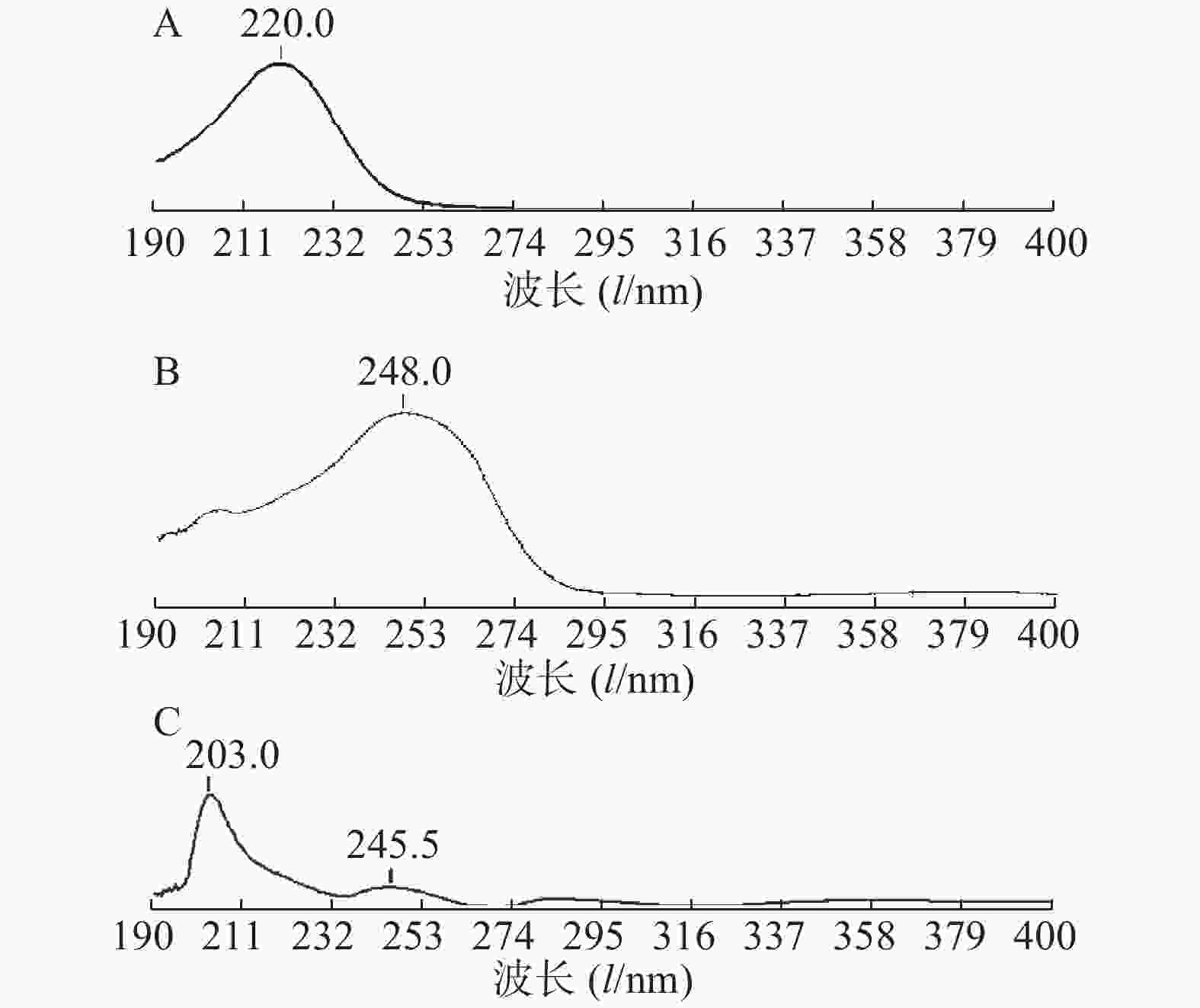
分别取酮康唑、莫匹罗星和糠酸莫米松适量,精密称定,加流动相制备成适宜浓度的溶液,以相应的溶剂为空白溶液,在190~400 nm波长范围内进行紫外扫描,结果见图1。
由图1可见,莫匹罗星在220 nm处具有最大吸收波长,糠酸莫米松在248 nm处具有最大吸收波长,酮康唑在203 nm和245.5 nm具有最大吸收波长,三者在220 nm与248 nm之间均有吸收,因在供试品中酮康唑和莫匹罗星的浓度均是糠酸莫米松的20倍,糠酸莫米松在220 nm处响应值较小,为保证3种药物能同时测定,确定酮康唑、莫匹罗星和糠酸莫米松的检测波长为248 nm。
-
色谱柱:Intersil ODS-3柱(250 mm×4.6 mm,5 µm),流动相为甲醇-pH5.5磷酸盐缓冲液(65∶35),柱温45 ℃,流速1.0 ml/min,检测波长248 nm,进样量10 µl。理论塔板数以各组分峰计,均不低于5000,各色谱峰的分离度良好。
-
酮康唑对照品溶液:取酮康唑20 mg,精密称定,置10 ml量瓶中,加入适量65%甲醇,超声使其完全溶解,加65%甲醇稀释至刻度,摇匀,得到酮康唑的标准储备液,4 ℃低温避光保存。精密吸取酮康唑的标准储备液1 ml,置于10 ml量瓶中,加65%甲醇稀释至刻度,摇匀,即得。
莫匹罗星对照品溶液:取莫匹罗星20 mg,精密称定,置10 ml量瓶中,加入适量65%甲醇,超声使其完全溶解,加65%甲醇稀释至刻度,摇匀,得到莫匹罗星的标准储备液,4 ℃低温避光保存。精密吸取莫匹罗星的标准储备液1 ml,置于10 ml量瓶中,加65%甲醇稀释至刻度,摇匀,即得。
糠酸莫米松对照品溶液:取糠酸莫米松10 mg,精密称定,置100 ml量瓶中,加入适量65%甲醇,超声使其完全溶解,加65%甲醇稀释至刻度,摇匀,得到糠酸莫米松的标准储备液,4 ℃低温避光保存。精密吸取糠酸莫米松的标准储备液1 ml,置于10 ml量瓶中,加65%甲醇稀释至刻度,摇匀,即得。
混合对照品溶液:取酮康唑、莫匹罗星和糠酸莫米松的标准储备液各1 ml,置10 ml量瓶中,加65%甲醇稀释至刻度,摇匀,即得。
-
取复方酮康唑软膏0.5 g,精密称定,置于50 ml的容量瓶中,加65%甲醇适量,超声溶解,加65%甲醇稀释至刻度,摇匀,即得供试品溶液。
-
取空白软膏基质0.5 g,精密称定,置于50 ml的容量瓶中,加65%甲醇适量,超声溶解,加65%甲醇稀释至刻度,摇匀,即得阴性对照溶液。
-
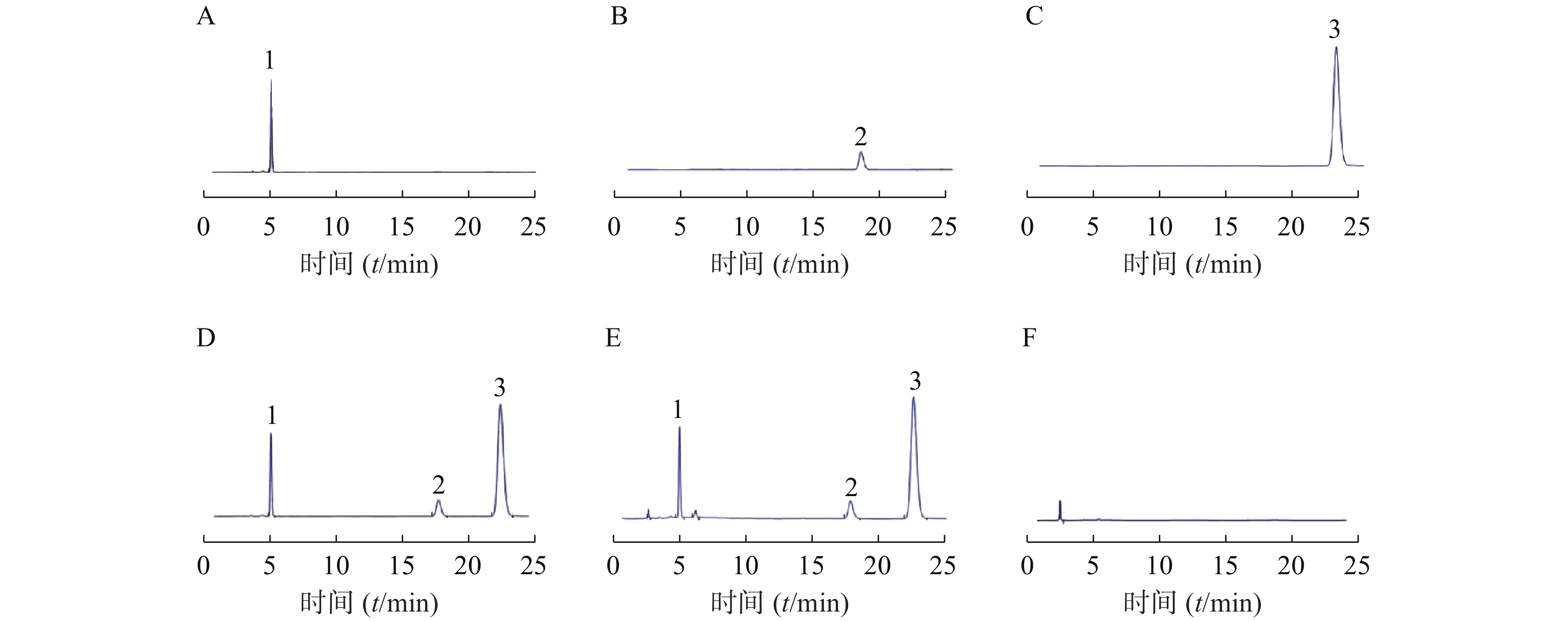
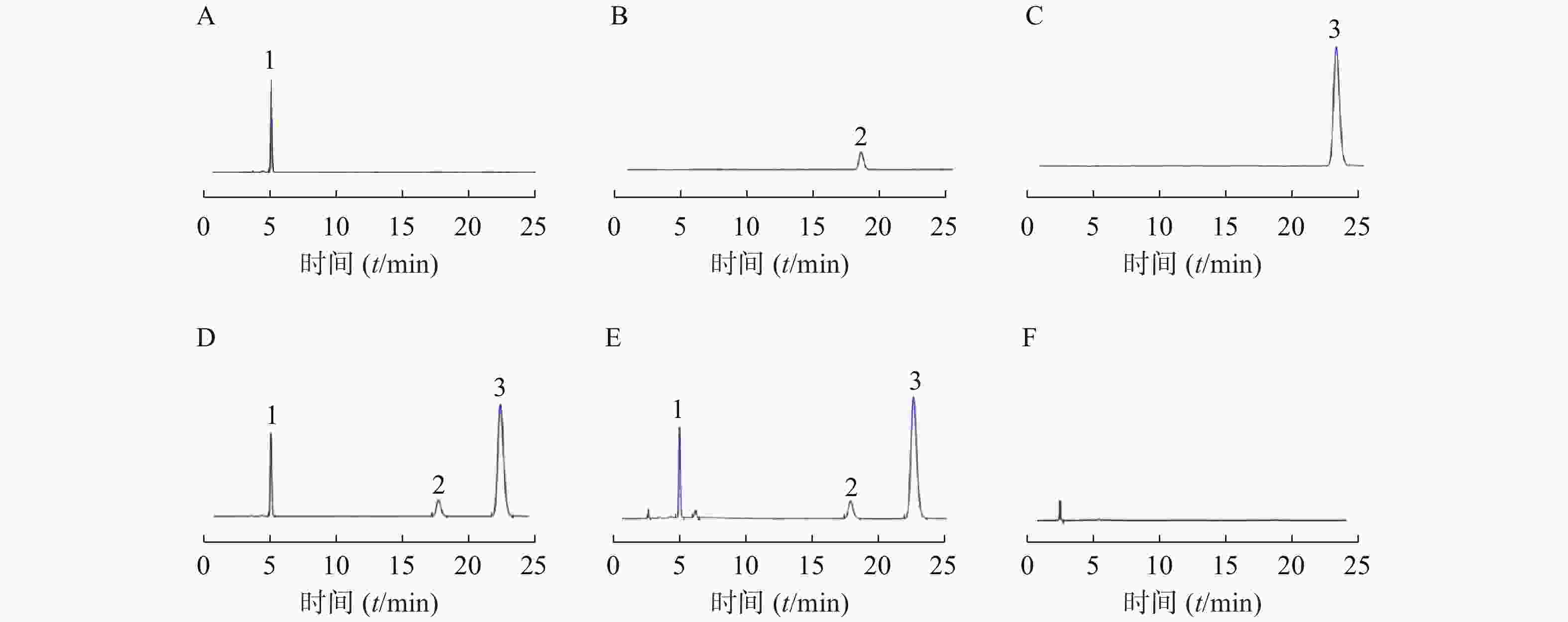
取上述对照品溶液、供试品溶液和阴性对照溶液,用0.22 μm微孔滤膜过滤,弃去初滤液,续滤液分别按照上述色谱条件进样,记录色谱图及相关参数。莫匹罗星的保留时间为5.075 min,理论塔板数为9196,对称因子0.81;糠酸莫米松的保留时间为18.413 min,理论塔板数为11859,对称因子0.88;酮康唑的保留时间为23.318 min,理论塔板数为12291,对称因子0.89,空白基质对莫匹罗星、糠酸莫米松和酮康唑的测定无干扰,方法专属性好。对照品溶液、供试品溶液及阴性对照溶液色谱见图2。
-
分别精密吸取酮康唑、莫匹罗星和糠酸莫米松标准储备液各0.2、0.4、0.8、1.0、1.2、1.4、1.6、2 ml,置10 ml量瓶中,用65%甲醇稀释至刻度,摇匀,0.22 μm微孔滤膜过滤,弃去初滤液,续滤液按上述色谱条件分别进样10 µl,记录色谱图峰面积。以峰面积A对浓度C (µg/ml)进行线性回归,结果见表1。
药名 线性方程 r 线性范围(µg/ml) 莫匹罗星 A=2.295C+10.20 0.9995 40.0~400.0 糠酸莫米松 A=28.240C+1.871 0.9995 2.0~20.0 酮康唑 A=12.280C+27.94 0.9995 40.0~400.0 -
分别精密量取同一批复方酮康唑软膏6份,每份约0.5 g,按“2.3.2”项下操作,测定,计算酮康唑、莫匹罗星和糠酸莫米松含量。结果见表2,结果表明该方法重复性良好。
药物 含量(µg/ml) 测得量(µg/ml) 平均含量(µg/ml) RSD(%) 莫匹罗星 200.00 203.60 204.30 1.51 200.00 204.90 200.00 201.70 200.00 208.80 200.00 206.40 200.00 200.40 糠酸莫米松 10.00 10.10 9.99 1.23 10.00 9.91 10.00 10.06 10.00 10.10 10.00 9.99 10.00 9.79 酮康唑 200.00 203.30 203.60 0.65 200.00 203.90 200.00 202.60 200.00 204.90 200.00 205.00 200.00 201.60 -
取供试品溶液,室温放置,分别于0、2、4、6、8、12、24 h进样10 µl测定,计算不同时间点莫匹罗星、糠酸莫米松和酮康唑的含量,结果见表3。
主药 时间(t/h) RSD
(%)0 2 4 6 8 12 24 莫匹罗星 100.00 99.94 100.00 99.94 99.81 99.12 98.49 0.68 糠酸莫米松 100.00 99.69 100.49 100.38 100.38 99.65 98.37 0.74 酮康唑 100.00 99.90 100.06 100.02 100.04 99.64 100.00 0.15 -
分别精密称取莫匹罗星8、10、12 mg,糠酸莫米松0.4、0.5、0.6 mg,酮康唑8、10、12 mg(相当于标示量的80%、100%、120%),精密称定,分别置于0.5 g的空白基质中,加适量流动相溶液,超声10 min使溶解,置于50 ml量瓶中,加流动相溶液稀释至刻度,摇匀,得低、中、高不同浓度的溶液,每个浓度各3份。用0.22 µm微孔滤膜过滤,弃去初滤液,续滤液按上述色谱条件分别进样,记录色谱图峰面积。根据回归方程计算出相应浓度和含量,并计算回收率、平均回收率及RSD。结果见表4。
药名 加入量(µg/ml) 测得量(µg/ml) 回收率(%) 平均回收率(%) RSD
(%)莫匹罗星 160.00 154.40 96.50 97.50 0.59 160.00 156.59 97.87 160.00 155.87 97.42 200.00 195.76 97.88 200.00 193.35 96.67 200.00 194.55 97.28 240.00 235.70 98.21 240.00 234.92 97.88 240.00 234.74 97.81 糠酸莫米松 8.00 7.73 96.62 97.99 0.79 8.00 7.84 98.00 8.00 7.83 97.91 10.00 9.88 98.80 10.00 9.71 97.10 10.00 9.78 97.80 12.00 11.88 99.00 12.00 11.85 98.75 12.00 11.75 97.92 酮康唑 160.00 153.63 96.02 97.62 0.74 160.00 156.39 97.74 160.00 155.84 97.40 200.00 195.72 97.86 200.00 194.06 97.03 200.00 195.44 97.72 240.00 236.46 98.52 240.00 235.60 98.17 240.00 235.38 98.08 -
取3批样品,依法测定,结果见表5。
样品批号 酮康唑 莫匹罗星 糠酸莫米松 20190411 99.03 99.20 101.4 20190415 106.3 99.76 101.6 20190408 100.6 100.7 100.7
2.1. 检测波长的确定
2.2. 色谱条件及系统适用性
2.3. 溶液的制备
2.3.1. 对照品溶液
2.3.2. 供试品溶液
2.3.3. 阴性对照溶液
2.4. 专属性考察
2.5. 线性关系考察
2.6. 重复性试验
2.7. 稳定性试验
2.8. 回收率试验
2.9. 样品含量测定
-
本研究根据软膏剂的特性,选择了提取效率较高,操作简便的超声提取法进行样品前处理,对溶剂种类、溶剂体积、提取时间进行考察,最终选择65%甲醇50 ml,超声提取10 min,该提取方法可有效的除去样品中的杂质,让测定的专属性更高。
-
对于流动相的选择,本实验尝试以甲醇-0.6%醋酸铵溶液作为流动相[9-12],结果基线非常不稳定,这可能是由于醋酸铵的紫外吸收所造成。以甲醇-水、乙腈-水,甲醇-磷酸二氢钠溶液和甲醇-乙腈-水等作为流动相[13-15],使用磷酸二氢钠溶液分离效果及峰形较好,有机相甲醇的比例应控制在一定范围,甲醇低于50%则酮康唑峰保留时间过长;流动相的酸度对酮康唑(弱碱性)和莫匹罗星(弱酸性)[16]的峰形及保留时间亦有影响,用磷酸将磷酸二氢钠的pH值调节到4.5、5.0、5.5和6.0。结果表明,当流动相的pH值为5.5时,莫匹罗星、糠酸莫米松和酮康唑的三组峰值均具有较好的分离度,并且无前延和拖尾现象。对于检测波长的选择,酮康唑、莫匹罗星和糠酸莫米松在220 nm与248 nm均有吸收,因在供试品中酮康唑和莫匹罗星的浓度均是糠酸莫米松的20倍,为了让这三种药物能同时测定,提高检测的灵敏度,确定最佳检测波长为248 nm。对于色谱柱的选择,本研究考察了岛津、安捷伦和沃特世等品牌的色谱柱,最终选择了岛津Intersil ODS-3柱,三种待测成分在该柱上分离度好,峰形佳,所以确定为最佳色谱柱。
-
3个批次的复方酮康唑软膏均为实验室自制,从测定结果可以看出,不同批次的样品中酮康唑、莫匹罗星和糠酸莫米松的含量有一定波动,这提示我们在进行中试放大生产时,要充分考虑各因素的影响,保证制剂中主要成分的含量稳定,同时对软膏剂的长期稳定性也需要进行考察。








 DownLoad:
DownLoad: