-
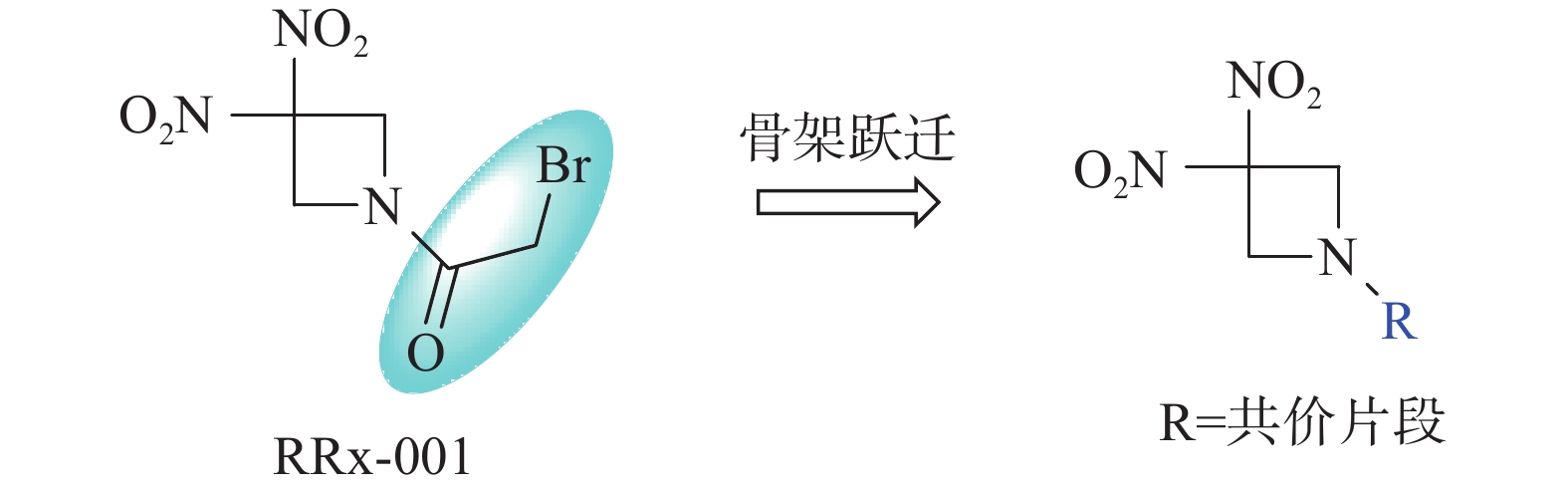
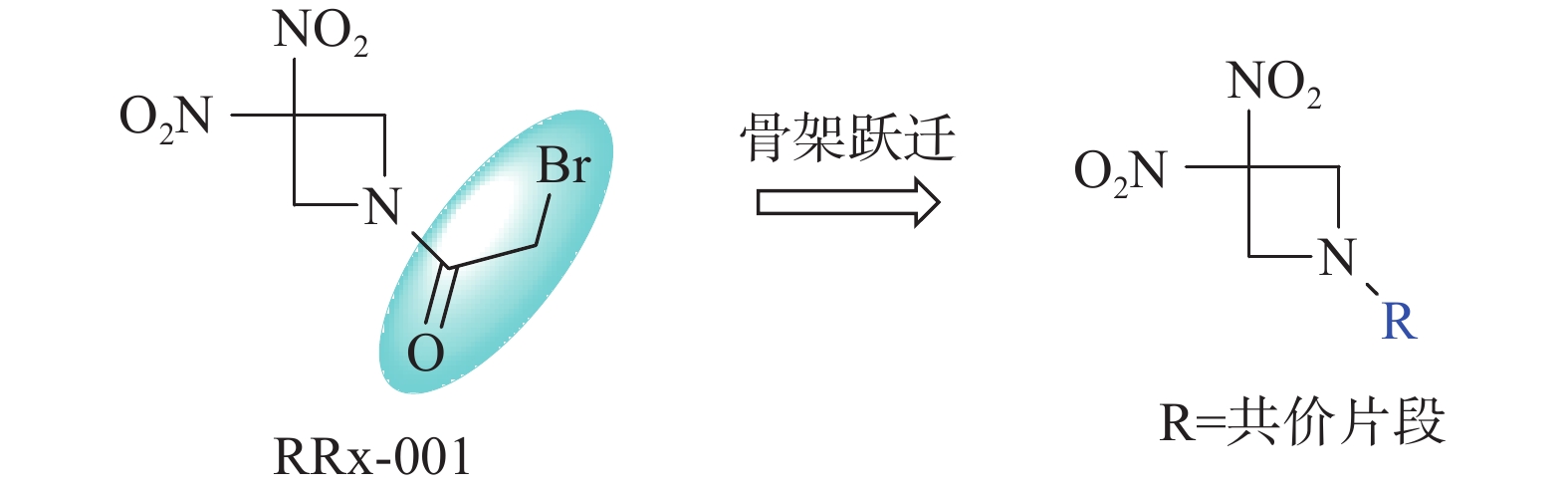
RRx-001是一种源于航空工业的肿瘤免疫治疗药物[1],其化学结构中含有溴代乙酰和双硝基氮杂环丁基团,目前处于III期临床试验[2]。RRx-001作为单一药物、放疗增敏剂或免疫增敏剂[3],用于细胞肺癌[4]、转移性结直肠癌[5]、卵巢癌[6]和胶质母细胞瘤[7]等肿瘤的治疗,显示出良好的疗效和安全性。研究发现,RRx-001具有多种作用机制,如靶向CD47-SIRPα信号通路,使肿瘤相关巨噬细胞复极化,由抗炎症M2表型转为促炎症M1表型[8];使肿瘤血管正常化,增加化疗药物渗透,产生代谢产物RONS,导致肿瘤细胞坏死[9];通过表观遗传抑制活性激活抑癌基因[10]等。作为靶向CD47的小分子药物,RRx-001在临床试验中未发现CD47抗体药物常见的嗜血综合征,表现出优于抗体大分子药物的安全性[11]。但RRx-001也存在明显的输注部位反应,因而临床需要采用特殊的注射器材,通过血液共同输注方式给药。
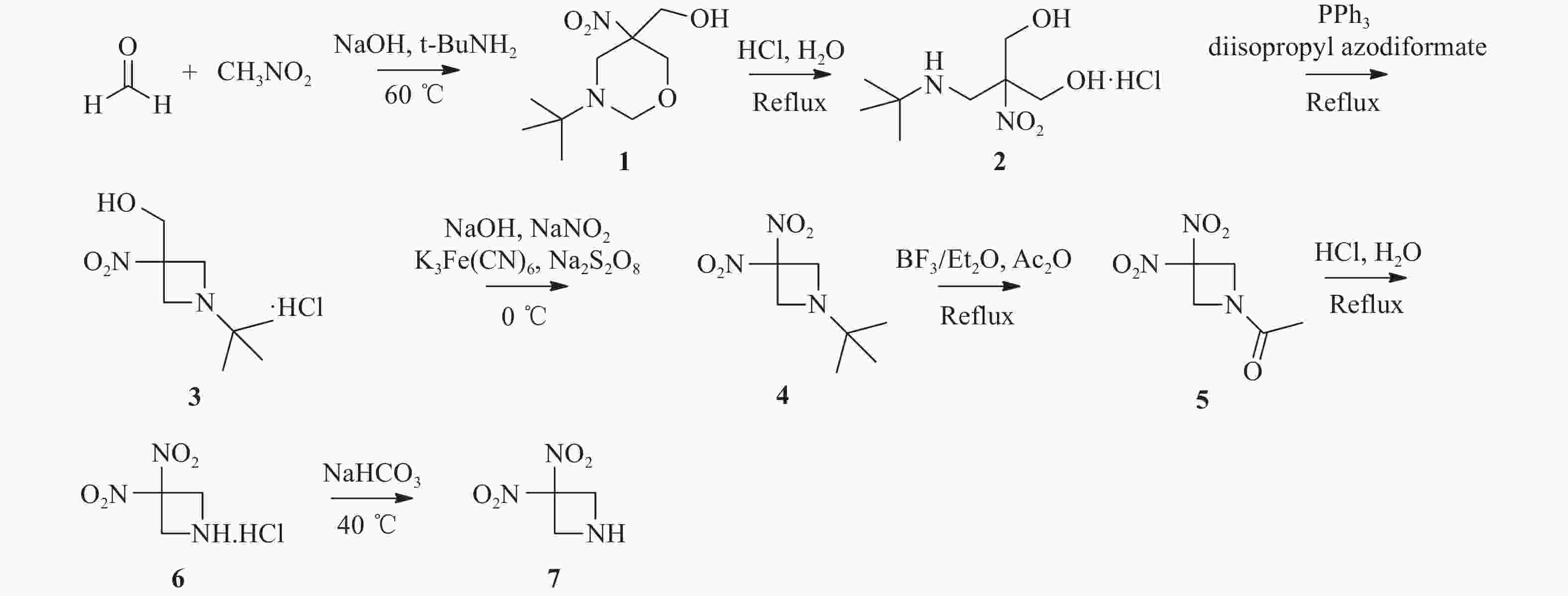
RRx-001结构中的溴代乙酰基为共价结合片段,也是其产生活性的药效基团[1]。为进一步探讨RRx-001的构效关系,本文拟采用骨架跃迁的药物设计策略,以不同的共价结合片段代替溴代乙酰基,分析其对抗肿瘤活性的影响,从而为后续基于RRx-001骨架的药物设计研究提供借鉴(图1)。
-
实验所用试剂分别采购自泰坦、毕得、乐研等公司,各类试剂均为市售分析纯或化学纯。核磁共振仪采用的是德国Bruke的600、400和300 MHz型,采用TMS作为内标,DMSO-d6、CDCl3或D2O等为溶剂。耦合常数(J)和化学位移(δ)单位分别用Hz和ppm来表示。高分辨质谱为德国Bruke micrOTOF
10257 ,抗肿瘤活性测试所用仪器为Biotek Synergy H2多功能酶标仪。薄层色谱(TLC)使用的硅胶板为GF254(中国青岛海洋化学),柱层析使用的是200~300目硅胶(中国青岛海洋化学)。 -
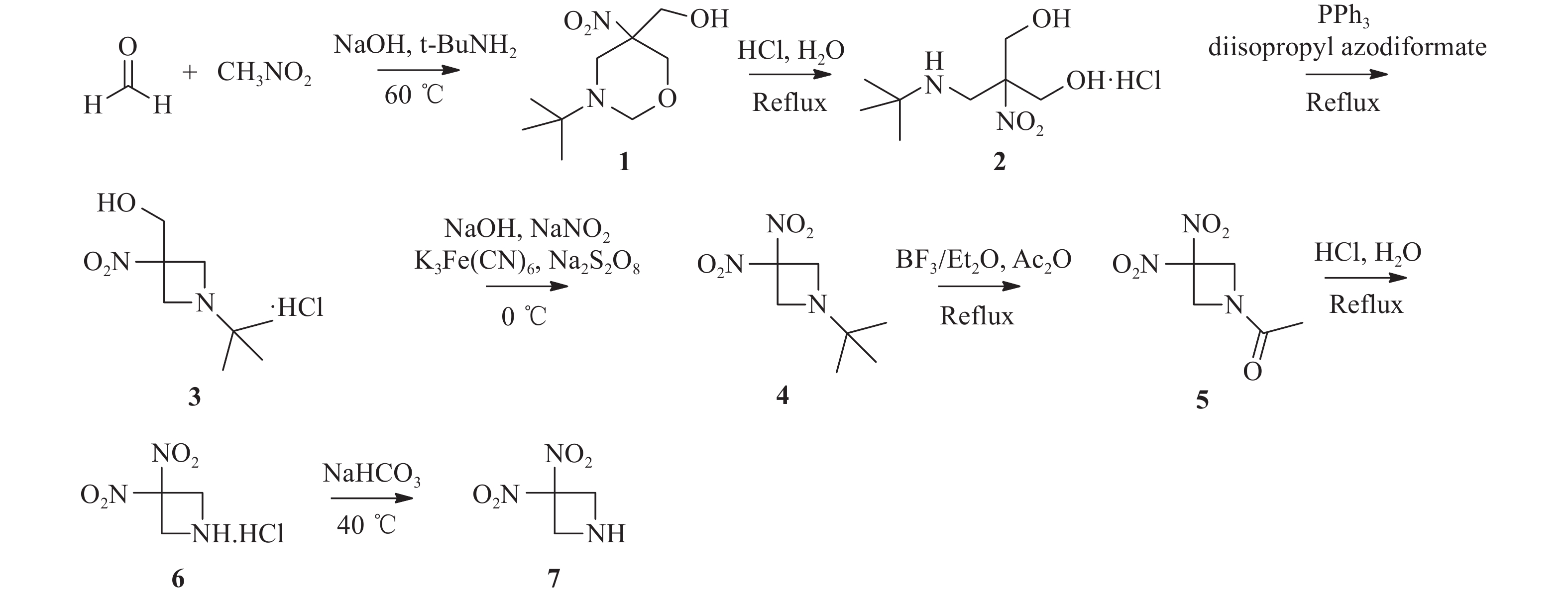
将多聚甲醛(24.1 g,0.8 mol)加入250 ml三颈烧瓶中,然后加入0.16 %氢氧化钠水溶液(40 ml),升温至40 ℃。缓慢滴加硝基甲烷(10.6 ml,0.2 mol),滴加结束后升温至60 ℃,滴加叔丁胺(21.2 ml,0.2 mol),滴加结束后继续反应10 min。反应液冷却至室温后过滤,水洗,干燥得38.8 g白色固体1,收率89.4 %,mp:136.1~138.2 ℃。1H NMR(400 MHz, DMSO-d6)δ:5.35(t, J=5.8 Hz, 1 H),4.42(t, J=10.0 Hz, 2 H),3.81(d, J=7.9 Hz, 1 H),3.63–3.51(m, 4 H),2.59(d, J=12.3 Hz, 1 H),0.95(s, 9 H)。
-
两颈烧瓶中依次加入500 ml无水乙醇、12 ml浓盐酸和化合物1(20.0 g,0.09 mol),加热回流反应6 h。反应结束后,减压蒸去溶剂,加入异丙醇搅拌0.5 h,过滤,洗涤,干燥得17.0 g白色晶体2,收率78.2 %,mp:175.5~178.3 ℃。1H NMR(400 MHz, D2O)δ:4.12(d, J=12.5 Hz, 2 H), 3.88(d, J=12.5 Hz, 2 H), 3.76(s, 2H), 1.36(s, 9 H)。
-
在氮气保护下,将化合物2(5.0 g,0.02 mol)加入到250 ml三颈烧瓶中,分别加入50 ml无水四氢呋喃和偶氮二甲酸二异丙酯(5.6 g,0.03 mol),升温至60 ℃。然后,将溶有三苯基磷(7.4 g,0.03 mol)的四氢呋喃溶液(15 ml)滴加到反应液中,滴加结束后继续反应5 h。反应液冷却至室温后过滤,洗涤,干燥得3.9 g白色固体3,收率86.6 %,mp:165.3~174.0 ℃。1H NMR(400 MHz, D2O)δ:4.74(s, 2 H), 4.43(s, 2 H), 4.16(s, 2 H), 1.26(s, 9 H)。
-
将化合物3(7.0 g,0.03 mol)和氢氧化钠水溶液(4.3 g,90 ml H2O)加入到250 ml两颈烧瓶中,室温搅拌2 h,冰浴冷却到10 ℃以下后,滴加亚硝酸钠(8.6 g,0.12 mol)、K3Fe(CN)6(1.0 g,0.003 mol)和水(5 ml)配成的溶液。控制反应温度为10~15 ℃下,分批次加入过硫酸钠(10.3 g,0.04 mol),然后升温至室温,反应过夜。反应液用20 ml二氯甲烷萃取3次,有机相用无水硫酸钠干燥,过滤,滤液减压蒸去溶剂得5.5 g黄色液体4,收率88.1 %。1H NMR(400 MHz, CDCl3)δ:4.12(s, 4 H), 1.05(s, 9 H)。
-
25 ml两颈烧瓶中依次加入化合物4(5.0 g,0.03 mol)、醋酸酐(9.8 ml,0.11 mol)和三氟硼酸乙醚溶液(0.5 ml,3.88 mmol),加热至120 ℃,回流反应12 h。减压蒸去溶剂,柱层析纯化(二氯甲烷 : 甲醇=100 : 1)得2.8 g淡黄色固体5,收率60.0 %,mp:110.1~113.9 ℃。1H NMR(600 MHz, CDCl3)δ:4.98(s, 2 H), 4.82(s, 2 H), 2.02(s, 3 H)。
-
将化合物5(1.0 g,5.29 mmol)加入到50 ml单颈烧瓶,然后加入6.3 ml 的5 %盐酸溶液,回流反应4 h。反应液冷却至室温,过滤,滤液减压蒸去溶剂得0.6 g黄色固体6,收率58.2 %,mp:150.3~155. 2 ℃。1H NMR(600 MHz, DMSO-d6)δ:4.98(s, 4 H), 3.42(s, 2 H)。
-
将化合物6(0.6 g,3.16 mmol)和15 ml水加入到50 ml两颈烧瓶中,升温至40 ℃,再滴加5 %碳酸氢钠溶液至pH为8。反应液用10 ml二氯甲烷萃取3次,有机相用无水硫酸钠干燥,过滤,减压蒸去溶剂得0.4 g黄色油状液体7,收率84.7 %。1H NMR(600 MHz, CDCl3)δ:4.51(s, 4 H), 2.20(s, 1 H)。
-
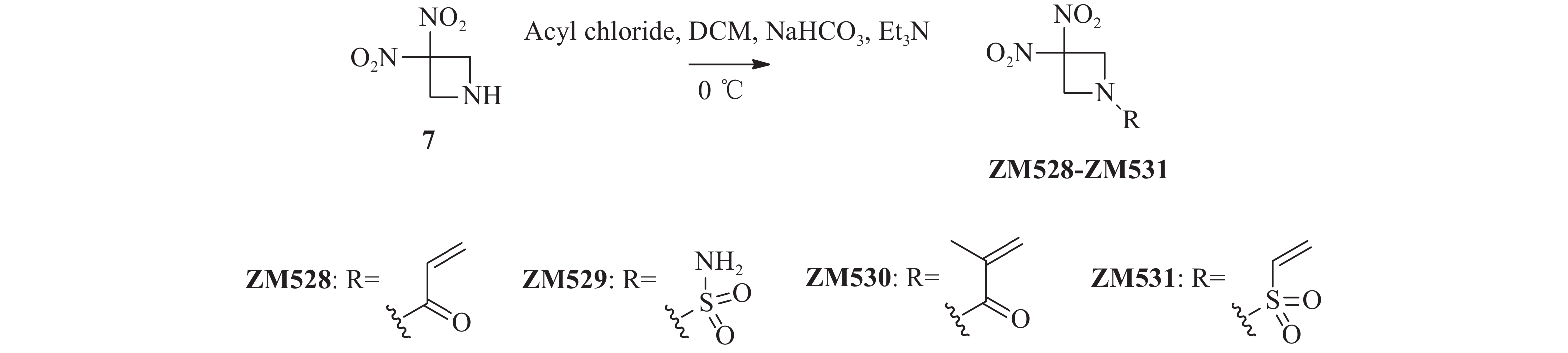
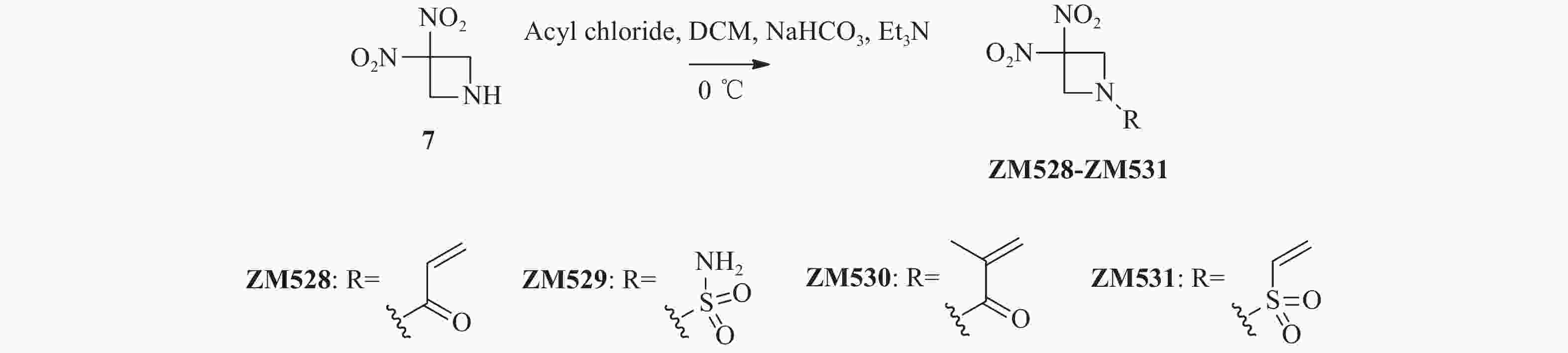
将关键中间体3, 3-二硝基氮杂环丁烷7(74.0 mg,0.50 mmol)加入到25 ml单颈烧瓶后,分别加入干燥的二氯甲烷(4 ml)和碳酸氢钠(42.0 mg,0.50 mmol),冰浴冷却至0 ℃后,滴加各种酰氯或磺酰氯(0.55 mmol),完全反应后减压蒸去溶剂,柱层析纯化(二氯甲烷 : 甲醇=100 : 1)得化合物ZM528~ZM531。
ZM528,白色固体,83 mg,收率61.3%。1H NMR(300 MHz, CDCl3) δ: 6.46(dd, J=16.9, 1.2 Hz, 1 H), 6.16(dd, J=16.9, 10.4 Hz, 1 H), 5.88(dd, J=10.4, 1.2 Hz, 1 H), 4.96(s, 4 H). HRMS(ESI, positive)m/z calcd for C5H7N3O5 [M + H]+:
202.0464 ; found 202.0458。ZM529,白色固体,77 mg,收率60.2%。1H NMR(300 MHz, DMSO-d6)δ: 7.47(s, 2 H), 4.77(s, 4 H). HRMS(ESI, positive)m/z calcd for C3H6N4O6S [M + H]+:
226.0008 ; found 226.0013。ZM530,白色固体,54 mg,收率50.1%。1H NMR(600 MHz, CDCl3)δ: 5.63–5.61(m, 1 H), 5.45(s, 1 H), 4.93(s, 4 H), 1.97(dd, J=1.6, 1.1 Hz, 3 H)。HRMS(ESI, positive)m/z calcd for C7H9N3O5 [M + H]+:
216.0620 ; found 216.0615。ZM531,淡黄色固体,81 mg,收率69.6%。1H NMR(600 MHz, CDCl3)δ: 6.58(dd, J=16.5, 9.8 Hz, 1 H), 6.46(d, J=16.6 Hz, 1 H), 6.27(d, J=9.8 Hz, 1 H), 4.75(s, 4 H)。
-
选用人结肠癌细胞HCT-116和人非小细胞肺癌细胞A549,于海军军医大学药物化学教研室冻存和传代。96孔板边缘每孔加入100 μl的PBS溶液防止边缘效应,内部每孔加入浓度为7×104个/ml的细胞悬液100 μl,置于37 ℃、5%二氧化碳培养箱内。24 h后,弃去96孔板内培养液,每孔分别加入100 μl受试化合物样品液和对照品液,设三复孔。将96孔板置于37 ℃、5%二氧化碳培养箱中培养72 h。实验采用CCK-8法[14-16]。在基础培养基中加入10 % CCK-8试剂制成混合液,弃去96孔板内旧培养基,加入混合液,100 μl/孔,将96孔板置于37 ℃、5 %二氧化碳培养箱中孵育2~4 h。使用酶标仪于450 nm波长处测定荧光OD值。细胞生长抑制率IC%=(空白对照孔OD值-给药孔OD值)/空白对照孔OD值×100%。根据各个浓度的IC%值,用GraphPad软件进行线性回归,算出各受试化合物抑制细胞生长50%的药物浓度,即IC50。
-
参考文献[12-13]设计了关键中间体3, 3-二硝基氮杂环丁烷的合成路线,合成路线见图2。以多聚甲醛和硝基甲烷为起始原料,通过环合、开环、Mistunobu、反向Henrry、脱叔丁基/乙酰化、脱乙酰基、碱化等7步反应合成出关键中间体7,总收率为14.9 %。其中,反向Henrry反应对催化剂的用量和反应温度较为敏感,经过反应条件优化,反应温度为10~15 ℃时,亚硝酸钠和K3Fe(CN)6的用量分别为化合物3的4倍和10%时,该步反应收率达88.1%,相比原有路线提升了6.3%。
将关键中间体7与各种酰氯或磺酰氯在三乙胺和碳酸氢钠催化下发生酰胺化反应,以较高收率合成得到目标化合物ZM528~ZM531(图3)。
-
采用CCK-8法测定目标化合物对肿瘤细胞株HCT-116和A549的体外抗肿瘤活性,以多柔比星(DOX)和RRx-001为阳性对照药,结果见表1。从表中可以看出,4个化合物均能保持一定的抗肿瘤活性,但相比RRx-001,均出现明显下降,其中丙烯酰基类化合物ZM528活性最高,对HCT-116和A549的IC50分别为(6.0±2.7)和(5.1±4.8)μmol/L。当丙烯酰基的2位引入甲基后活性明显下降,以磺酰基代替酰基后,活性也呈现下降趋势。以磺酰胺基代替丙烯酰基后,对HCT-116和A549肿瘤细胞株的活性分别下降了6.4和5.4倍,但当以磺酰基代替磺酰胺基后活性基本保持。以上研究结果初步表明,RRx-001的溴代乙酰基对抗肿瘤活性影响较大,以其他共价结合片段代替后活性出现明显下降。
化合物IC50(μmol/L) HCT-116细胞株 A549细胞株 RRx-001 0.94±0.7 <0.2 ZM528 6.0±2.7 5.1±4.8 ZM529 38.1±19.7 27.3±10.4 ZM530 >50 14.4±3.0 ZM531 34.5±13.5 36.3±5.3 DOX <0.2 <0.2 -
采用骨架跃迁药物设计策略,设计合成出4个RRx-001衍生物。体外抗肿瘤活性研究发现,所有新化合物对HCT-116和A549细胞株的抑制活性均显示出明显的下降,但能保持一定的抗肿瘤活性,其中丙烯酰基类化合物ZM528活性最高,对两种肿瘤细胞株的IC50分别为(6.0±2.7)和(5.1±4.8) μmol/L,其原因可能是RRx-001的共价结合片段为溴乙酰基,结合活性高于其他共价结合片段。初步构效关系显示,氮杂环丁烷的氨基上溴代乙酰基取代后活性最高,丙烯酰基取代活性次之。研究结果和初步构效关系为以RRx-001为骨架的新型靶向CD47药物的进一步优化设计研究提供了理论指导。
Synthesis and Antitumor Activity of Novel RRx-001 Derivatives
doi: 10.12206/j.issn.2097-2024.202408053
- Received Date: 2024-08-30
- Rev Recd Date: 2025-03-06
-
Key words:
- RRx-001 /
- immuno-oncology /
- covalent inhibitor /
- synthesis /
- antitumor
Abstract:
| Citation: | WU Ruonan, TANG Wenmin, GAO Lin, WU Yuelin, LUO Chuan, MIAO Zhenyuan. Synthesis and Antitumor Activity of Novel RRx-001 Derivatives[J]. Journal of Pharmaceutical Practice and Service. doi: 10.12206/j.issn.2097-2024.202408053 |







 DownLoad:
DownLoad: