-
抗真菌感染尤其深部真菌感染是抗微生物感染的重要研究内容,也是全球范围医学研究中面临的严峻挑战。近年来,在免疫力低下和重症监护病房患者中[1],真菌感染发病率和病死率逐年增加,其中在侵袭性念珠菌病中,病死率约为40%[2]。白色念珠菌是真菌感染中最常见的病原体,可以在正常人体的皮肤、口腔、阴道和肠道等部位定植[3]。临床上,多烯类、棘白菌素类、唑类和烯丙胺类药物是治疗真菌感染最常用的药物[4],然而由于白色念珠菌的耐药性、形成生物被膜以及部分药物较大的毒副作用等问题,传统抗真菌药物的应用面临新的挑战[5]。因此,增加现有抗真菌药物的药效,降低其毒副作用,是抗真菌药物研发的重要方向。利用药物新剂型的研究降低其毒副作用是有效的药物研发途径。例如,成功上市的两性霉素B脂质体即可极大降低两性霉素B的肾脏毒性[6]。
醋酸卡泊芬净(CAS)是一种棘白菌素半合成类抗真菌药,作用于真菌细胞的细胞壁,能有效抑制细胞壁中β-1,3-D-葡聚糖的生物合成,是目前白色念珠菌系统性感染治疗中的一线推荐药物。然而,CAS价格昂贵,通过药物合用的方式,维持或增强CAS的治疗效果,降低其使用剂量,可以显著减少患者的用药成本。与此同时,降低药物剂量可以减少CAS的毒副作用。有研究显示,患者在接受高于批准剂量3倍的CAS作用下,高剂量组中65%的患者会出现肝毒性,包括肝酶升高和肝衰竭[7]。我们在前期研究中发现单硬脂酸甘油酯(GMS)与CAS联合用药具有良好的协同作用,增强CAS体外抑菌效果,如两者联用体外抑菌MIC由
0.0625 μg/ml降低到0.0313 μg/ml[8]。然而,GMS与CAS混合使用,由于溶解性差异和药代动力学不同,可能无法在体内同时达到作用部位,共同杀灭真菌,本研究通过制备醋酸卡泊芬净单硬脂酸甘油酯固体脂质纳米粒为上述问题解决提供思路,并发挥体内协同抗菌的效果。固体脂质纳米颗粒(SLNs)以固态天然或合成的类脂如卵磷脂、甘油三酯等为载体,将药物包裹或夹嵌于类脂核中制成的纳米给药系统[9],可以包载亲脂性或者亲水性药物,提高药物的稳定性[10]。具有毒性低生物相容性好、物理稳定性好、体循环中药物不易泄露的特点[11]。通过药物协同增加抑真菌作用,可以减少给药用量与给药次数,提高药物疗效;进而降低抗真菌药物的毒副反应;同时减少真菌耐药性的产生[12]。基于以上研究基础,本研究以单硬脂酸甘油酯作为载体材料制备卡泊芬净固体脂质纳米粒,考察纳米粒对卡泊芬净的协同增效作用。
-
标准菌株白色念珠菌SC5314由美国Georgetown大学William A Fonzi教授赠予。
-
醋酸卡泊芬净(上海源叶)(≥98%);单硬脂酸甘油酯、蛋白胨、葡萄糖、琼脂(上海生工);乙腈、泊洛沙姆188、磷酸、甲醇(中国国药);DMEM高糖培养基、PBS缓冲液(上海泰坦);酵母提取物、营养肉汤(BD公司);RPMI
1640 (美国Gibco)。马尔文粒度电位仪(英国Malvern);透射电子显微镜(日本JEOL);高效液相色谱仪(美国Thermo);超声波细胞粉碎机(宁波新芝生物);高速离心机(德国Hettich);涡旋混合器(日本LTS);电子天平(瑞士MettlerToledo);洁净工作台(上海力申);多功能功能酶标仪(瑞士TECAN)。
-
ICR小鼠,雌性,体重20~22 g,由苏州华创信诺医药科技有限公司提供,动物许可证号:SCXK(苏)2020-0009。
-
将在SDA固体培养基上保存的白色念珠菌单克隆菌株转接到3 ml YEPD培养基,30 ℃,200 r/min,培养18 h,使白色念珠菌处于指数生长的平台期。将活化的菌株转移到离心管中,重悬、离心洗涤3次后加入1 ml pH=7.4 PBS(0.01 mol/L)重悬,备用。
-
色谱柱:Diamonsil Plus C18柱(4.6 mm×250 mm,5 μm);流动相:乙腈∶0.1%磷酸(35∶65);流速:1 ml/min;检测波长:227 nm;柱温为25 ℃;进样量:20 μl。
-
专属性考察:取醋酸卡泊芬净标准液、空白SLNs溶液、CAS-SLNs溶液,经0.22 μm滤膜过滤后,按“2. 2. 1”项下色谱方法进样检测。
线性和范围:精密称取CAS并定量配置质量浓度为1、5、10、20、40、50 μg/ml的CAS溶液。按“2.2.1”项下色谱方法进行检测,横坐标为CAS浓度,纵坐标为峰面积,绘制标准曲线。
精密度:日内精密度取5、20、40 μg/ml 3个浓度的CAS溶液各3份,分别检测3次;日间精密度是取低、中、高3个浓度的CAS溶液各3份,每3 d进行检测。
溶液稳定性:同一样品在0、1、2、3、5、8、12 h分别按“2.2.1”项下色谱方法进行检测,计算RSD以考察样品溶液的稳定性。
回收率:取空白SLNs溶液,分别取5、20、40 μg/ml 3个浓度的CAS溶液,超声破乳后,经0.22 μm滤膜过滤后按“2. 2. 1”项下色谱方法进行检测,计算回收率。
-
采用熔融法制备醋酸卡泊芬净固体脂质纳米粒(CAS-SLNs):称取处方量的CAS、GMS、卵磷脂(CAS、GMS、卵磷脂摩尔比为1∶20∶5)完全溶解于甲醇中,加热条件下磁力搅拌混合均匀作为油相。配置0.2%泊洛沙姆188溶液,加热至相同温度作为水相。在磁力搅拌条件下(500 r/min),将水相缓慢滴入油相,加热条件下持续搅拌30 min,得到初乳。取初乳超声10 min(250 W,1 S,1 S),4 ℃固化过夜,即得到CAS-SLNs。
-
马尔文粒度仪测定SLNs的粒径和Zeta电位,磷钨酸负染后透射电镜观察其表面形态。
-
使用低温超速离心法,取所制备的CAS-SLNs溶液于离心管内
15000 r/min下离心2 h,取上清液500 μl于5 ml量瓶内并定容至刻度。另取500 μl未经离心的CAS-SLNs溶液于5 ml量瓶内并定容至刻度,甲醇超声破乳,分别取以上各组溶液经0.22 μm滤膜进样检测并计算载药量和包封率。根据公式(1)计算包封率(EE)、公式(2)计算载药量(DL): -
制备药敏实验板:取96孔板。第1列配置100 μl RPMI1640培养基做空白对照;第12列配置100 μl菌液做阳性对照;第2列加200 μl菌液,分组为CAS、CAS + GMS(CAS与GMS物理混合)、CAS+SLNs(CAS与空白SLNs物理混合)、CAS-SLNs,每组两行,每孔CAS含量浓度为0.313 μg/ml。2~11列进行倍比稀释,最终使得CAS浓度为3.13×10−2 μg/ml、1.57×10−2 μg/ml、7.83×10−3 μg/ml、3.91×10−3 μg/ml、1.96×10−3 μg/ml、9.78×10−4 μg/ml、4.89×10−4 μg/ml、2.45×10−4 μg/ml、1.23×10−4 μg/ml、6.13×10−5 μg/ml、3.06×10−5 μg/ml。30 ℃恒温孵育24 h,用酶标仪在λ=630 nm处测A值。100 μl RPMI 1640培养基做空白对照记为A空白,菌液做阳性对照记为A阳性,加入各实验组记为A实验,MIC定义为使白色念珠菌A630降低80%以上的最小药物浓度。
-
制备生物被膜板:1640培养基调节白色念珠菌浓度为3×106 CFU/ml。取96孔板,第1列加100 μl RPMI1640培养基做空白对照;第2至12列加100 μl菌液,37 ℃培养90 min。
制备药敏板:分组为CAS、CAS+GMS、CAS+SLNs、CAS−SLNs,每组两行,每孔CAS含量浓度为0.313 μg/ml。按“2.4.1”项下倍比稀释得到不同浓度的CAS。
生物被模板孵育90 min后,弃上清液,用PBS清洗,将药敏板中的CAS按浓度梯度分别加入第2至11列,37 ℃培养24 h。倒掉培养基,PBS清洗生物被膜,加入0.4%结晶紫水溶液染色。倒掉染液并用PBS冲洗,加入酒精脱色后,取适量上清至96孔板,用酶标仪在λ=630 nm处,测A值。生物被膜板可在显微镜下观察被膜形成情况。
-
选择ICR小鼠雌性,6~8 周龄,小鼠尾静脉注射SC5314。注射剂量为1×105 CFU/只。1 d后处死小鼠,收集肾脏组织进行PAS染色切片。小鼠体重检测:将实验动物分为对照组、CAS组、CAS-SLNs组,每组8只。注射白色念珠菌后,分别尾静脉注射生理盐水、CAS、CAS-SLNs,每只200 μl(0.2 mg/kg)。间隔24 h给药,连续给药3次。给药后连续观察20 d,每天记录小鼠体重(g)。
-
实验动物造模后随机分为3组:对照组、CAS组、CAS-SLNs组。模型建立后分别尾静脉注射生理盐水、CAS、CAS-SLNs,每只200 μl(0.2 mg/kg)。感染48 h后处死,将肾脏组织称重后置入PBS中研磨成匀浆,将组织匀浆稀释到合适的倍数,接种到SDA平板上,在30 ℃下培养48 h后进行菌落计数。
-
实验动物分为对照组、CAS组、CAS-SLNs组,模型建立后分别尾静脉注射生理盐水、CAS、CAS-SLNs。感染48 h后处死,并取其肾组织进行PAS染色切片,观察白色念珠菌在组织中的感染情况。
-
采用GraphPad Prism 8(GraphPad Software, San Diego, CA)计算。两组间数据的比较采用Unpaired Student’s t-test,多组间的数据比较采用One-way ANOVA。P>0.05表示差异不具有统计学意义,P<0.05表示差异显著,P<0.01表示差异非常显著,P<0.001表示差异极其显著。
-
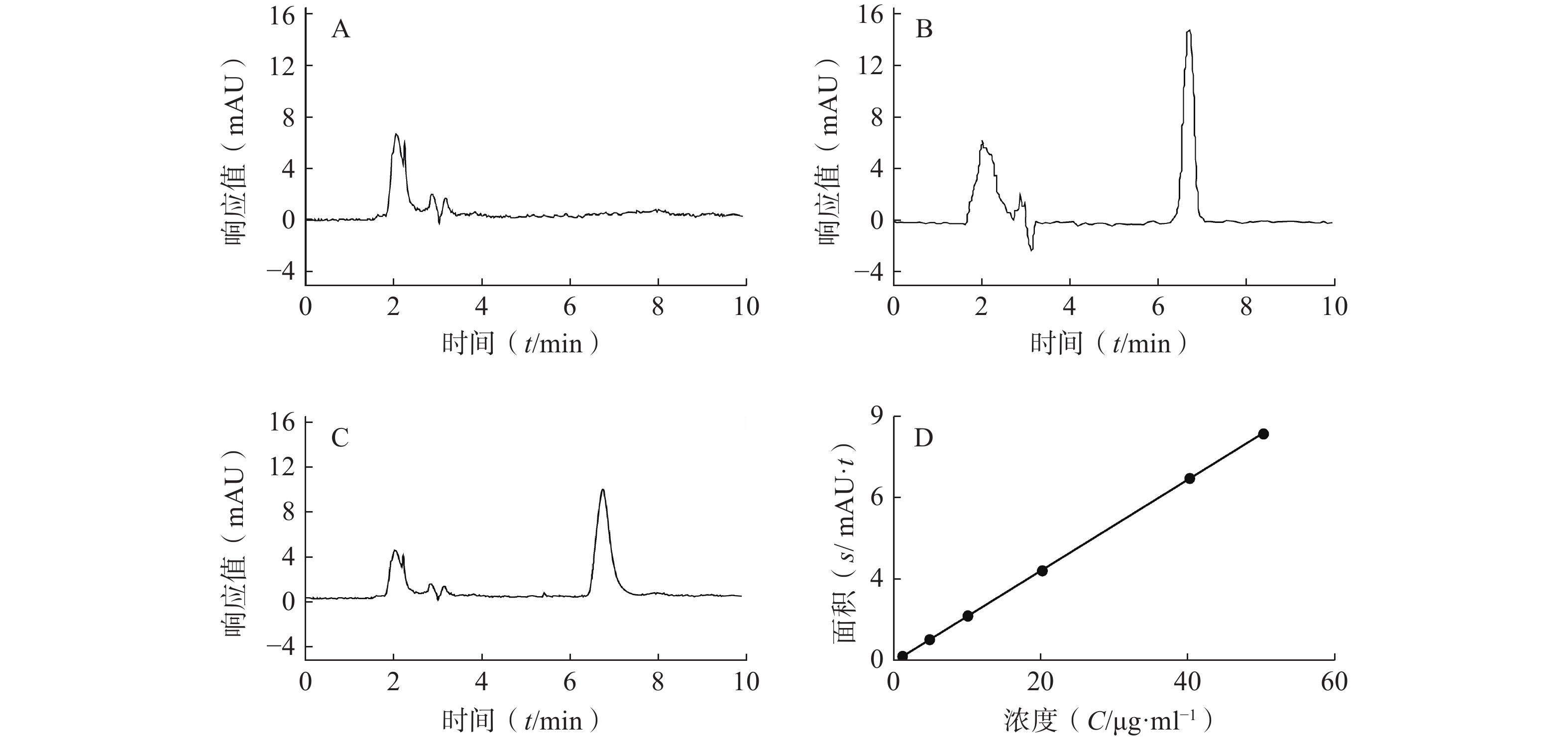
专属性:液相色谱图见图1,CAS出峰位置处无SLNs色谱法干扰,建立方法专属性良好。CAS保留时间为6.8 min,载体对CAS-SLNs测定无干扰。
线性和范围:如图1所示,方法专属性良好,以CAS浓度(C, μg/ml)为横坐标,所测得峰面积(Area)为纵坐标绘制标准曲线,回归方程为A=0.168 7C−
0.038 1 ,R2=0.999 9 ,表明CAS在1~50 μg/ml内线性关系良好。精密度:由表1可知,低、中、高浓度CAS日内精密度和日间精密度RSD值均小于5%,表明所建立方法的精密度满足要求。
加样量(μg/ml) 日内精密度 日间精密度 测得量(μg/ml) RSD(%) 测得量(μg/ml) RSD(%) 5 4.79±0.05 1.07 4.79±0.04 0.90 20 19.64±0.17 0.87 19.90±0.26 1.32 40 38.48±0.21 0.55 38.17±0.57 1.49 稳定性:同一样品在12 h以内的RSD为1.88%,表明含量测定供试品溶液的稳定性满足要求。
回收率:由表2可知,样品在低、中、高3个浓度的回收率在95%~115%范围内,且RSD值均小于5%,表明该方法稳定可靠,可用于样品中CAS含量的测定。
加样量(μg/ml) 测得量(μg/ml) 平均回收率(%) RSD(%) 5 5.29±0.12 105.78±2.41 2.28 20 20.95±0.48 104.76±2.40 2.29 40 43.39±0.59 108.48±1.47 1.35 -
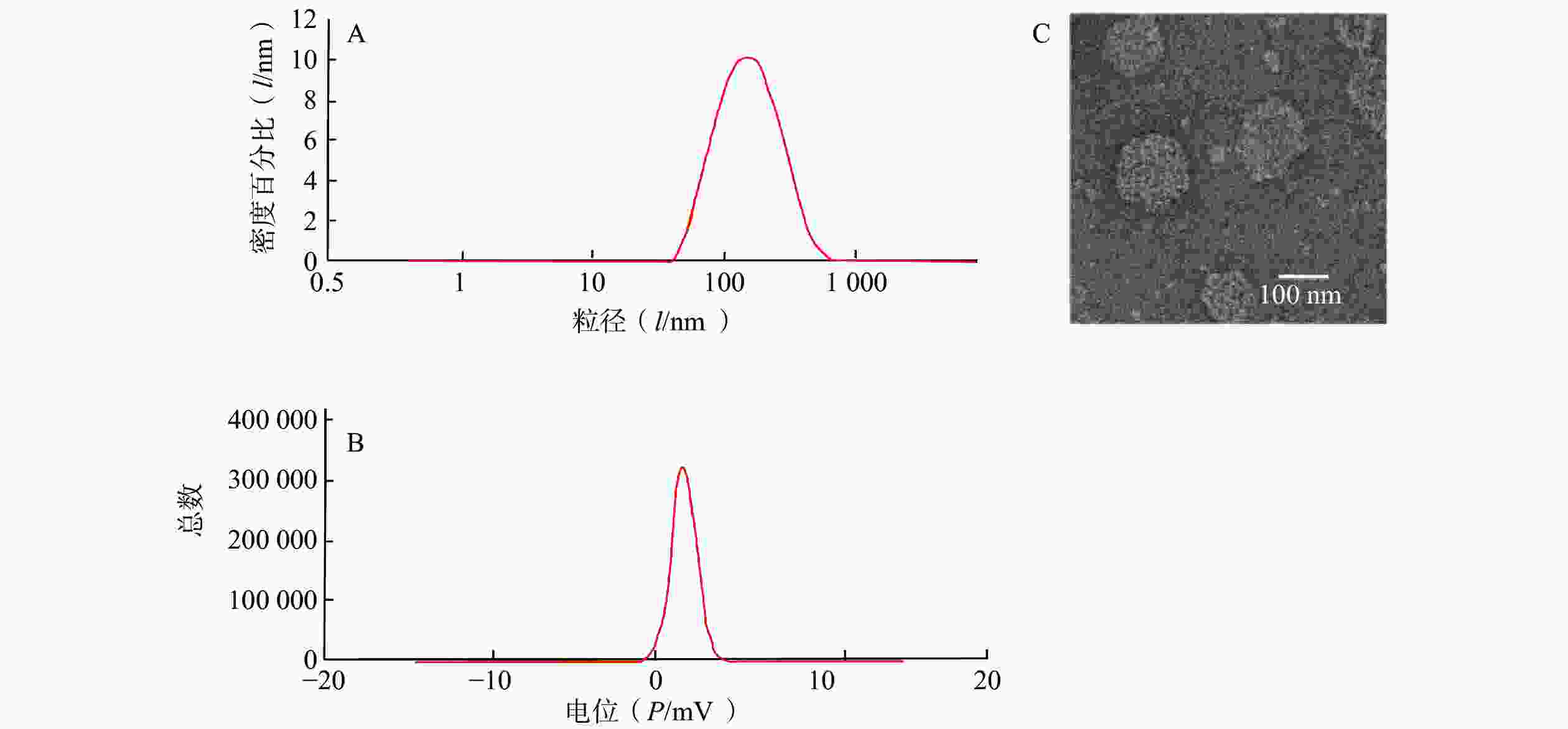
制备CAS-SLNs并对其进行表征。CAS-SLNs的粒径、Zeta电位分别为(135.97±1.73)nm,(19.33±0.37)mV。如图2所示,透射电镜照片显示CAS-SLNs为类圆形粒子,粒径约为120 nm。纳米粒包封率为(67.71±1.74)%,载药量为(7.55±0.68)%。
-
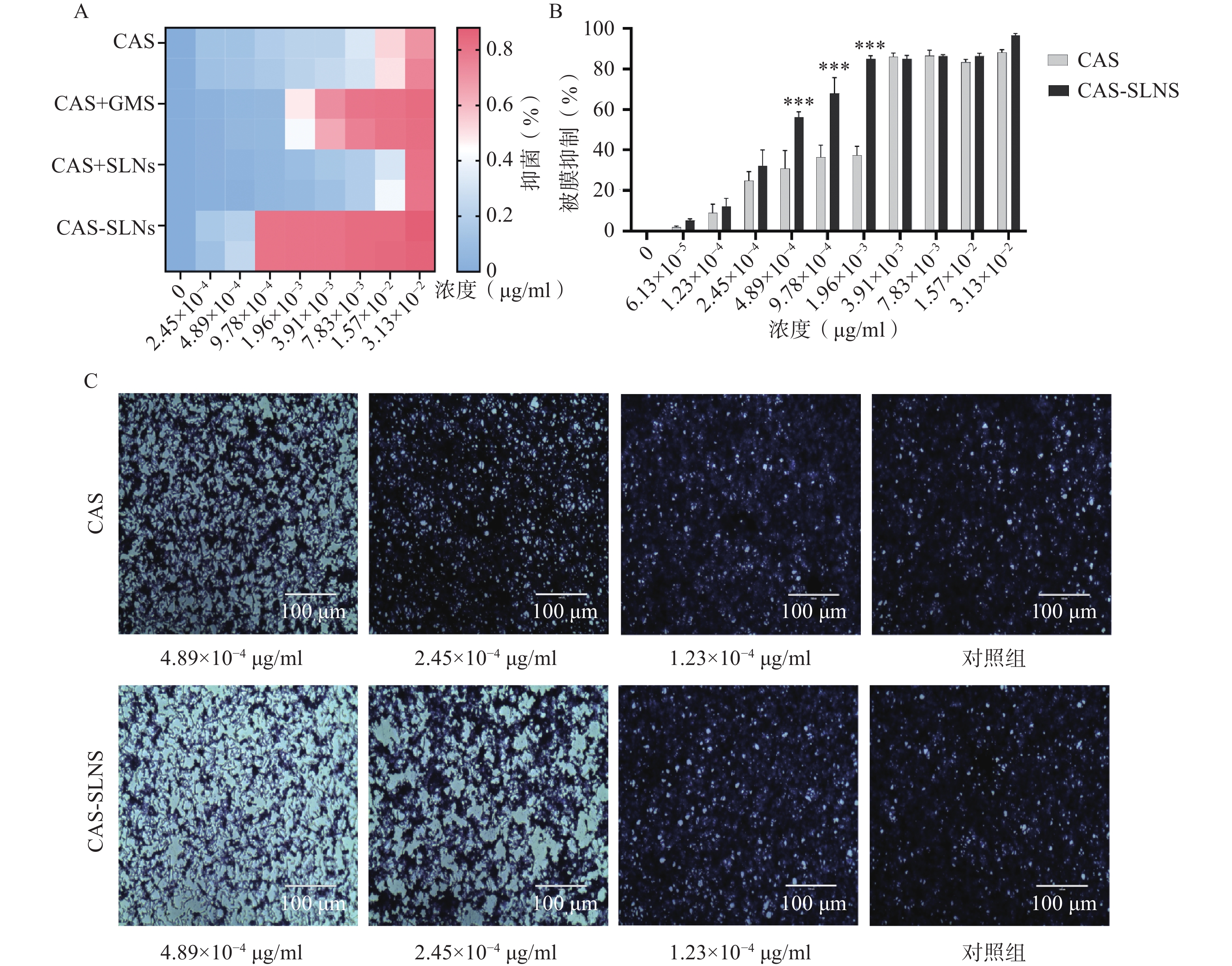
前期本课题组研究表明,GMS可以协同CAS抑制白色念珠菌的生长,因此我们比较了CAS-SLNs与CAS单用以及CAS+GMS合用的体外抗真菌活性。
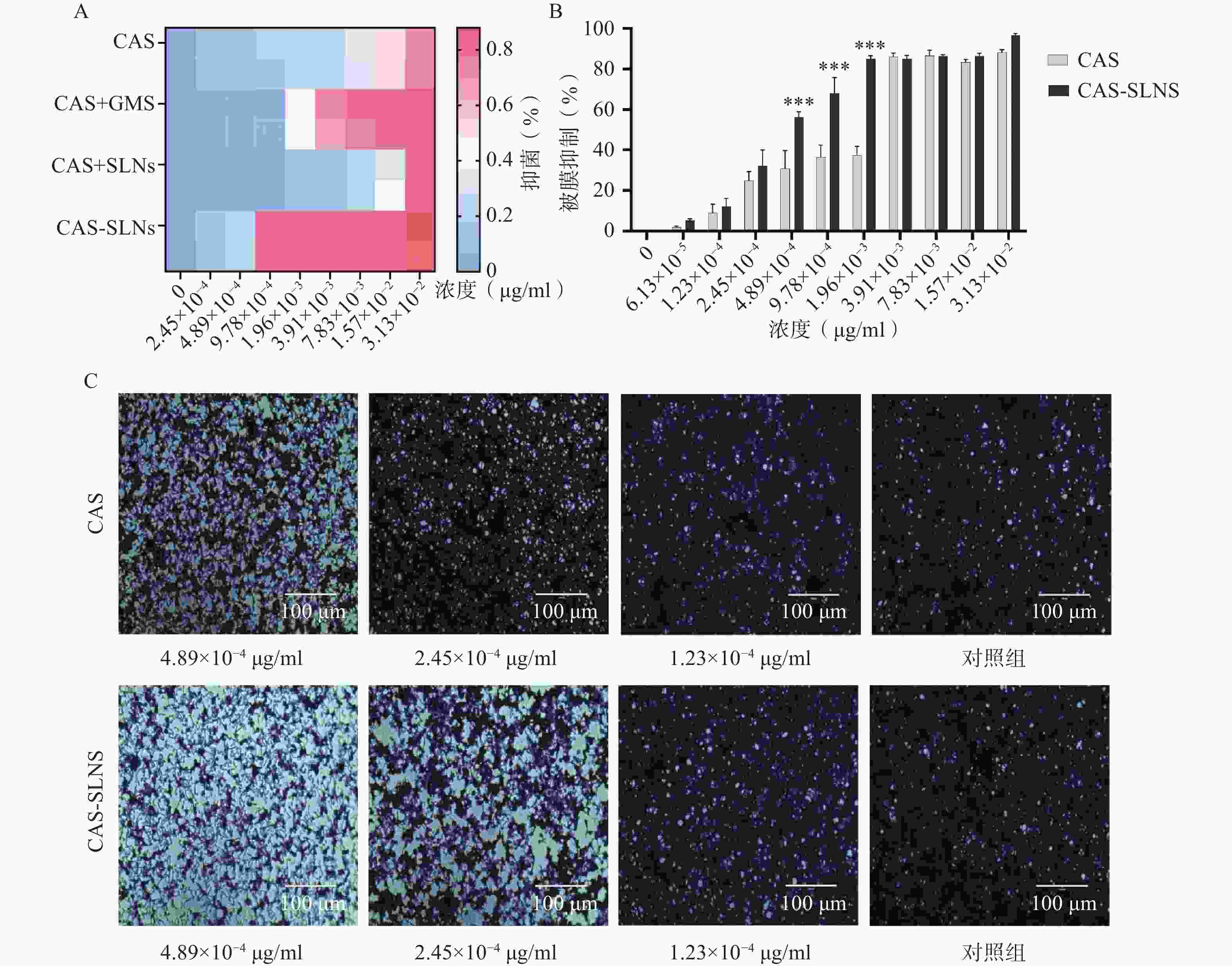
结果如表3所示,与1 μg/ml的GMS合用后,CAS的MIC从3.13×10−2 μg/ml降低到7.83×10−3 μg/ml,白色念珠菌对CAS的敏感性增加了4倍,表明CAS可以协同GMS抑制念珠菌的生长;而本课题制备的CAS-SLNs MIC为9.78×10−4 μg/ml,这与单用CAS的MIC 3.13×10−2 μg/ml相比,活性增强了32倍,这表明CAS-SLNs可以进一步发挥CAS与GMS的协同作用抑制念珠菌的生长,其抑菌活性性优于CAS和GMS物理混合组。
菌株名称 CAS CAS+GMS CAS+SLNs CAS-SLNs C. albicans SC5314 3.13×10−2 7.83×10−3 3.13×10−2 9.78×10−4 -
菌丝态与酵母态混合形成的被膜是白色念珠菌耐药的重要因素,卡泊芬净单用可以显著抑制白色念珠菌被膜的形成,为了探究CAS-SLNs的优效性,采用结晶紫染色法定量检测了各组药物处理后白色念珠菌生物被膜的形成情况。结果如图3显示,CAS组在1.96×10−3、9.78×10−4、4.89×10−4 mg/ml浓度下的被膜形成抑制率分别为(37.30±4.40)%、(36.50±5.78)%、(30.70±8.96)%,CAS-SLNs组在同等的1.96×10−3、9.78×10−4 、4.89×10−4 mg/ml浓度下,被膜形成的抑制率分别为(85.03±1.59)%、(68.05±7.72)%、(56.12±2.83)%。由此可见CAS-SLNs对生物被膜形成的抑制作用与单用CAS相比显著增强。
-
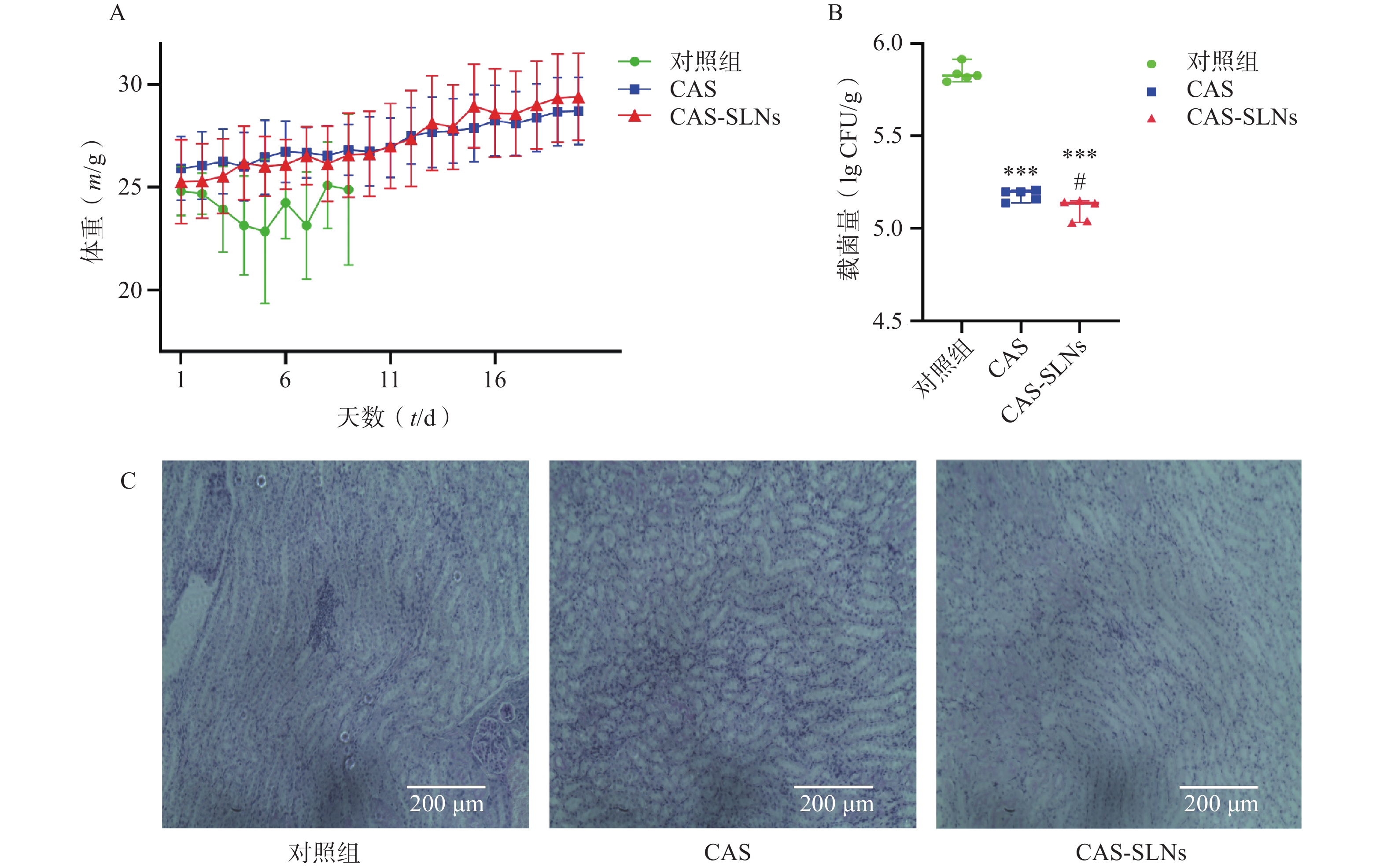
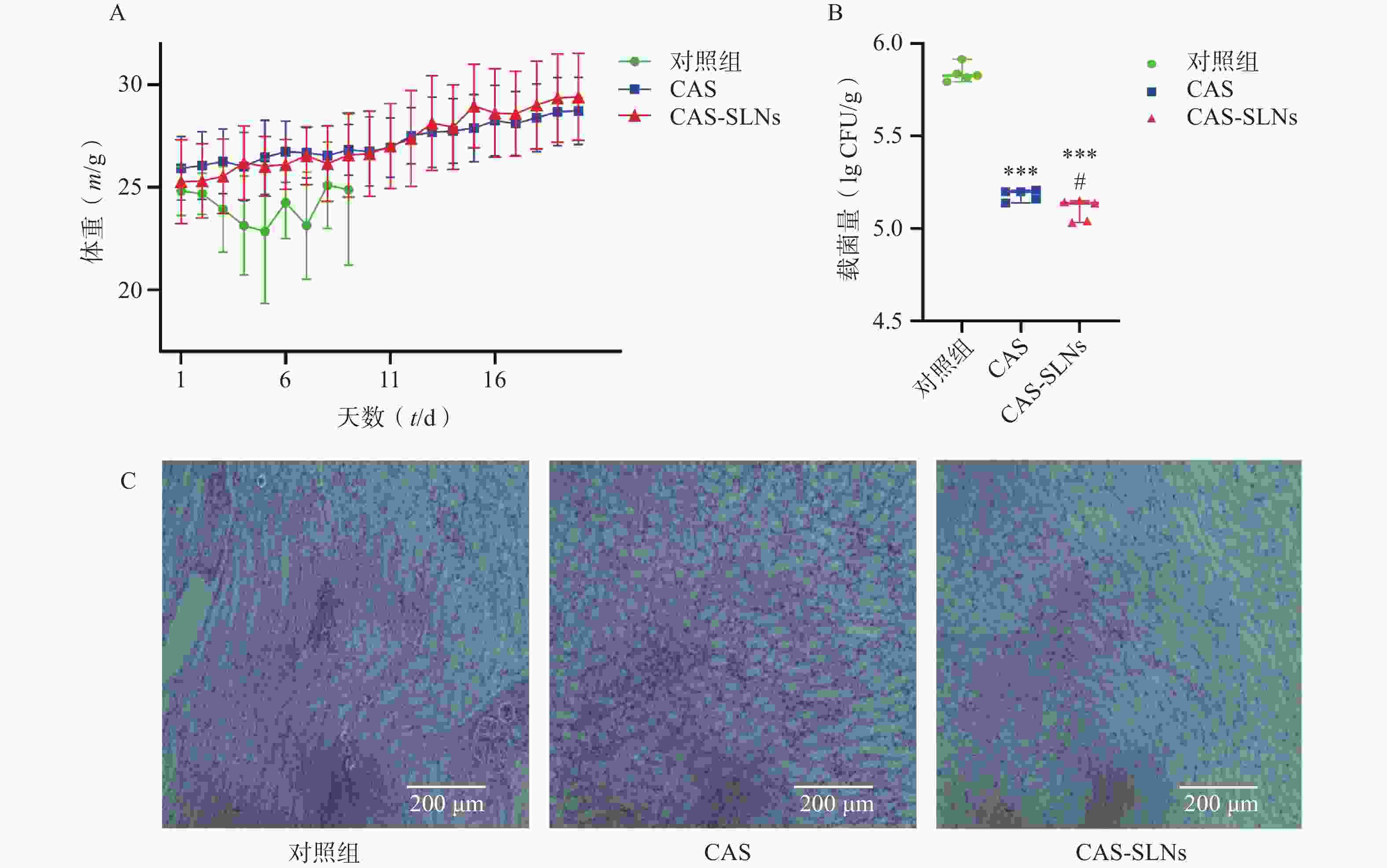
在小鼠系统性白色念珠菌感染模型中,如图4A所示,白色念珠菌感染可以导致小鼠体重的下降,而药物治疗可以恢复小鼠的体重增长。结果显示,CAS组小鼠平均体重由25.93 g增长至28.73 g,增长10.8%;而CAS-SLNs组体重由25.29 g增长至29.43 g,体重增长16.36%;对照组(对照组第10天存活率为0)与CAS-SLNs有非常显著的差异(P<0.01),CAS-SLNs组体重恢复更加显著。此外,CAS-SLNs治疗也可以显著降低小鼠肾脏的载菌量。如图4B所示,对照组、CAS组、CAS-SLNs组的载菌量分别为(5.84±0.05)CFU/g、(5.18±0.03)CFU/g、(5.10±0.06)CFU/g,与对照组肾脏载菌量有极显著差异(P<0.001),CAS-SLNs组与CAS组也有显著性差异(P<0.05)。通过PAS染色,考察小鼠肾脏的白色念珠菌感染情况,如图4C所示,在SC5314感染小鼠48 h后,对照组肾部病理切片经过PAS染色出现大量白色念珠菌。在感染小鼠经过CAS治疗后,白色念珠菌的数量明显下降。而经过同等剂量的CAS-SLNs治疗的感染小鼠肾部组织切片很少发现白色念珠菌的存在。CAS-SLNs作为以GMS为主要材料的载体包载药物CAS,其抗菌效果优于单用CAS,可以在体内发挥CAS与GMS的协同作用,体内抗菌效果显著增强。
-
侵袭性真菌感染每年导致超过150万人死亡[13]。由于抗真菌药物耐药性的不断增多,迫切需要新的策略来对抗危及生命的真菌疾病。白念珠菌是念珠菌病的主要病原体,针对念珠菌血症的初始抗真菌治疗,美国传染病学会(IDSA)指南推荐使用棘白菌素类药物[14]。然而,抗真菌药物可能在毒性、感染复发、高成本和出现抗真菌耐药性方面存在局限性,可以采用联合用药的方式克服上述问题[15]。研究结果显示,在替代疗法中同时使用尼可霉素Z和CAS或米卡芬净[16]、以及CAS和氟康唑或伏立康唑的联用可以更好的治疗念珠菌感染[17];此外,宿主防御肽模拟物brilacidin(BRI)也可以作为CAS的增效剂,增强CAS对烟曲霉、白念珠菌、耳念珠菌和固有抗性的新型隐球菌的抗菌活性[18]。我们在前期研究发现CAS与GMS具有较强的协同抗念珠菌活性[8],GMS作为一种惰性的药用辅料,之前已报道过其作为脂质基质制备SLNs发挥协同抗金葡萄球菌和大肠埃希菌的活性[19],但抗真菌活性未有报道。因此,本研究构建了以GMS为主要材料的固体脂质纳米粒作为纳米载体包载CAS。一方面,SLNs骨架材料GMS可以协同CAS发挥抗真菌增效作用,另一方面,本课题所制备的纳米粒为水分散系统,无须有机溶剂,符合临床使用的需要。本研究结果表明CAS-SLNs相对于CAS与GMS物理混合物,MIC从7.83×10−3 μg/ml降低到9.78×10−4 μg/ml,发挥了很好的增效作用,这可能与CAS-SLNs的粒径为纳米级,具有极大的比表面积,容易被真菌摄取有关。在局部微环境中,随着载体基质的降解,药物逐步释放,在局部形成了较长时间的协同抑菌环境,从而增加了药物的抑菌活性。在可能的机制方面,有研究显示,硬脂酸可以激活三酰基甘油合成代谢途径,影响真菌的脂质代谢[20]。而棘白菌素类药物作用于白念珠菌后,白念珠菌脂质代谢的相关基因会发生改变,提示脂质代谢可能在白念珠菌抵御CAS的杀伤过程中十分重要[21]。因此,我们推测CAS-SLNs可能通过改变脂质代谢的途径,提高了CAS的敏感性,具体的信号通路和作用机制需要进一步的通过转录组学、代谢组学等方式深入研究。本研究为后续卡泊芬净的剂型改造提供了新的思路,有望为解决临床卡泊芬净药物治疗成本高的问题提供新的研究方向。
Study on the synergistic antifungal effects of caspofungin acetate loaded glyceryl monostearate nanoparticle on Candida albicans
doi: 10.12206/j.issn.2097-2024.202310043
- Received Date: 2023-10-22
- Rev Recd Date: 2024-05-30
- Available Online: 2025-03-14
- Publish Date: 2025-03-25
-
Key words:
- Candida albicans /
- caspofungin acetate /
- glyceryl monostearate solid lipid nanoparticles /
- synergistic antifungal
Abstract:
| Citation: | GUO Lingyi, LIU Yanchao, GAO Lu, LIU Ruiyao, LYU Quanzhen, YU Yuan. Study on the synergistic antifungal effects of caspofungin acetate loaded glyceryl monostearate nanoparticle on Candida albicans[J]. Journal of Pharmaceutical Practice and Service, 2025, 43(3): 136-142, 150. doi: 10.12206/j.issn.2097-2024.202310043 |







 DownLoad:
DownLoad: