-
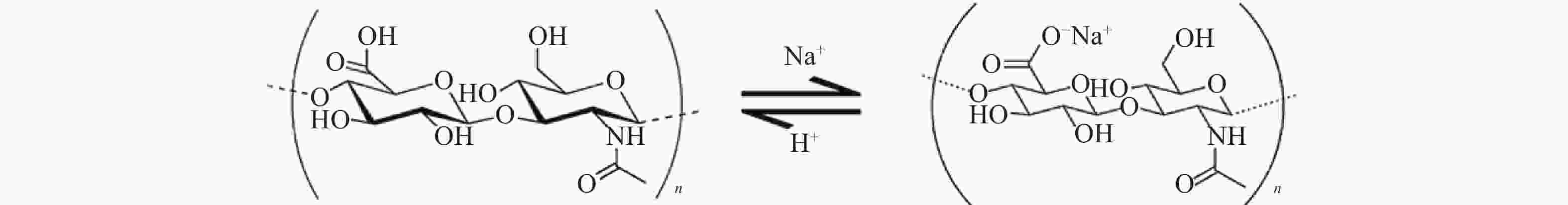
透明质酸钠(图1)又称玻璃酸钠,是由交替的N-乙酰葡糖胺(GlcNAc)和葡糖醛酸(GlcA)单元组成[1]的聚合物,聚合度最大的相对分子质量(Mr)可以达到107。具有高度黏弹性、可塑性、渗透性、独特的流变学特性以及良好的生物相容性等特点[2]。透明质酸钠广泛存在于人体,是构成人体细胞间质、眼玻璃体、关节滑液等结缔组织的主要成分,在体内具有保水、维持细胞外空间、调节渗透压、润滑、促进细胞修复等重要生理功能[3]。医用透明质酸钠凝胶(HA)为无色透明黏稠液体,主要成分为透明质酸钠。HA常用于肿瘤患者术后的抗粘连,其广泛的生理学活性有可能使得肿瘤细胞易于扩增和转移,文献报道内源性的透明质酸能促进肿瘤增殖[4-6]。因此,本研究考察外源性HA是否会促进肿瘤的生长和转移,以明确HA是否可用于肿瘤患者的术后防粘连。为HA用于肿瘤患者腹、盆腔手术术后防粘连提供实验依据。
HTML
-
医用透明质酸钠凝胶(HA,10 mg/ml,上海昊海生物科技股份有限公司,批号:18052122,Mr≥1.0×106),氟尿嘧啶注射液(5-Fu,上海旭东海普药业有限公司,批号:FA1800416),胎牛血清(FBS,Gibco,批号:1932595),McCoy’s 5A Medium(Gibco,批号:1967679),RPMI Medium 1640(Gibco,批号:1967664),MEM(Minimum Eagle’s Medium,HyClone,批号:AD20532472),胰酶(Trypsin,上海博光生物科技有限公司),磷酸盐缓冲液(PBS,上海博光生物科技有限公司),MTT(上海博光生物科技有限公司),无水甲醇、二甲基亚砜(DMSO,国药集团化学试剂有限公司),生物胶(批号:217201N2)、小鼠结肠癌细胞(CT26,上海中西医结合医院),人宫颈癌细胞(Hela)、人结肠癌细胞(HCT116,中国科学院上海生命科学研究院)。
-
裸鼠:88只,上海斯莱克实验动物有限公司,SPF级,SCXK(沪)2017-0005;64只,上海灵畅生物科技有限公司,SPF级,SCXK(沪)2018-0003),动物饲养于同济大学沪北校区(SYXK(沪)2018-0034)。所有动物实验过程均符合实验动物伦理学要求。
-
酶标仪1510-00411C(Thermo),坤宏BMB224分析天平(南京伯尼塔科学仪器有限公司),ECLIPSE TE100 显微镜(Nikon),细胞培养箱(Thermo)。
1.1. 实验材料
1.2. 实验动物
1.3. 实验仪器
-
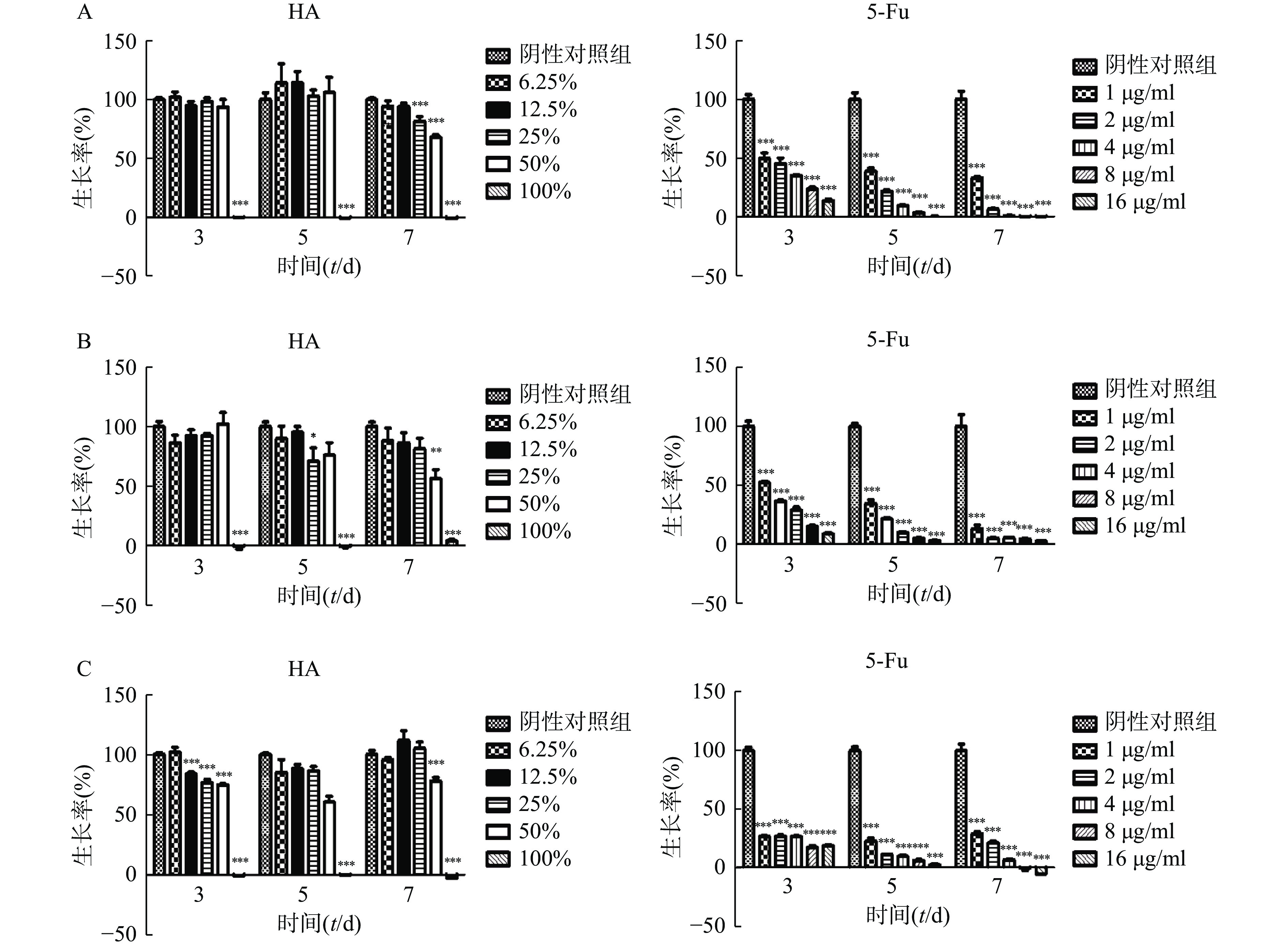
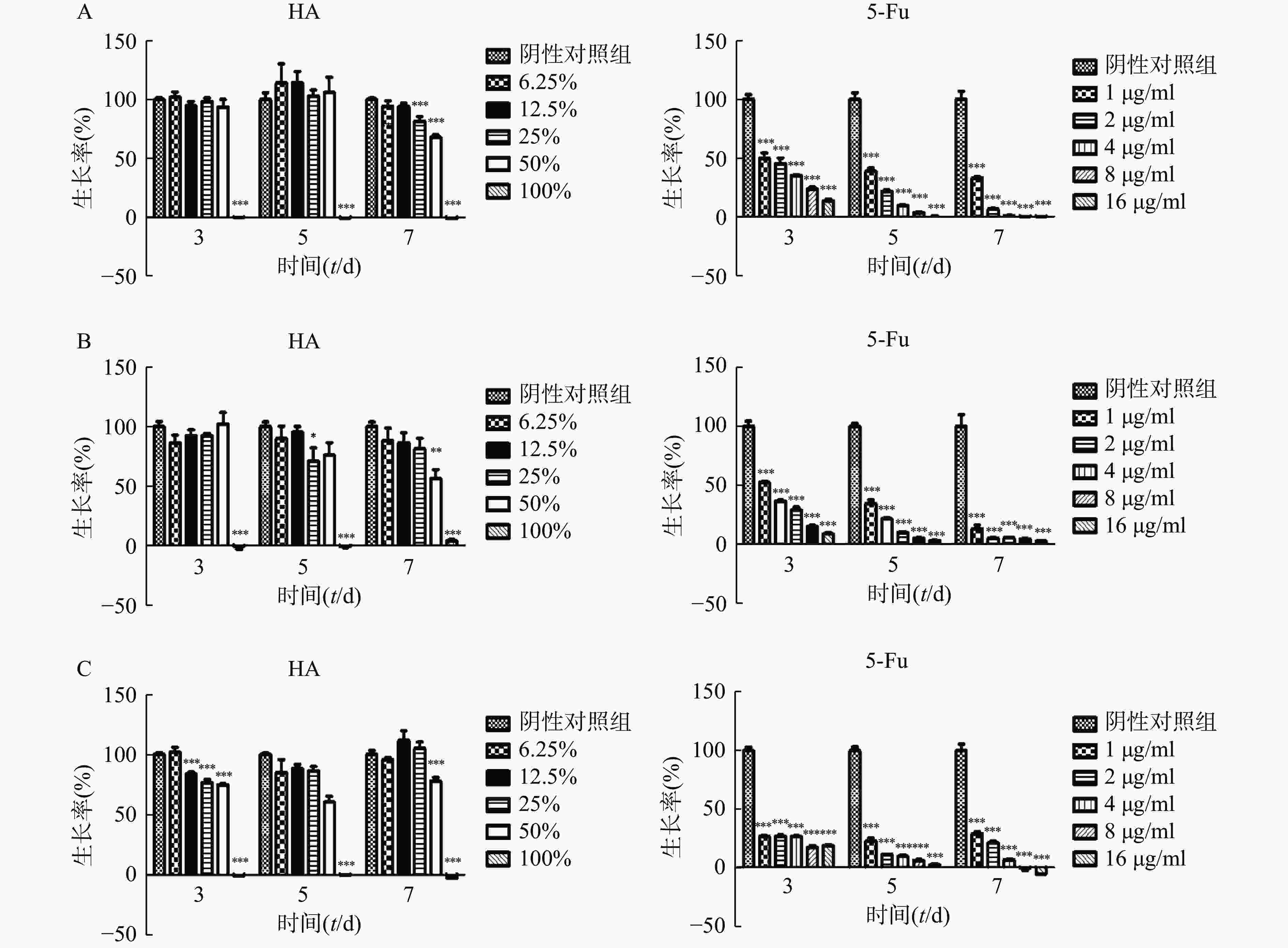
将状态良好的肿瘤细胞用胰酶消化后离心,弃上清液,分别用对应培养液重悬成单个肿瘤细胞悬液,显微镜下计数,然后分别稀释成以下浓度:Hela(2×104个/ml),CT26(5×103个/ml),HCT116(104个/ml)。取96孔板,空白对照孔接入100 μl培养液,其余各孔接入肿瘤细胞混悬液,每孔100 μl,将96孔板置于37 ℃、5%CO2培养箱培养。24 h后吸弃培养液,空白对照和阴性对照孔分别加入200 μl培养液,测试孔分别加入100%HA、50%HA+50%培养液、25%HA+75%培养液、12.5%HA+87.5%培养液、6.25%HA+93.75%培养液,每孔200 μl;阳性对照孔分别加入5-Fu浓度为 16、8、4、2、1 μg/ml培养液,每孔200 μl,将加药96孔板放回培养箱培养。分别培养3、5和7 d 后,吸弃上层液体,加入含0.5 mg/ml MTT的培养液100 μl,培养4 h。避光处吸弃上层液体,每孔加入100 μl DMSO,避光反应10 min,振荡1 min,在酶标仪波长570 nm处(630 nm校准)测量各孔的吸光值。细胞存活率=(实验孔吸光值—空白对照孔吸光值)/(阴性对照孔吸光值—空白对照孔吸光值)×100%。
-
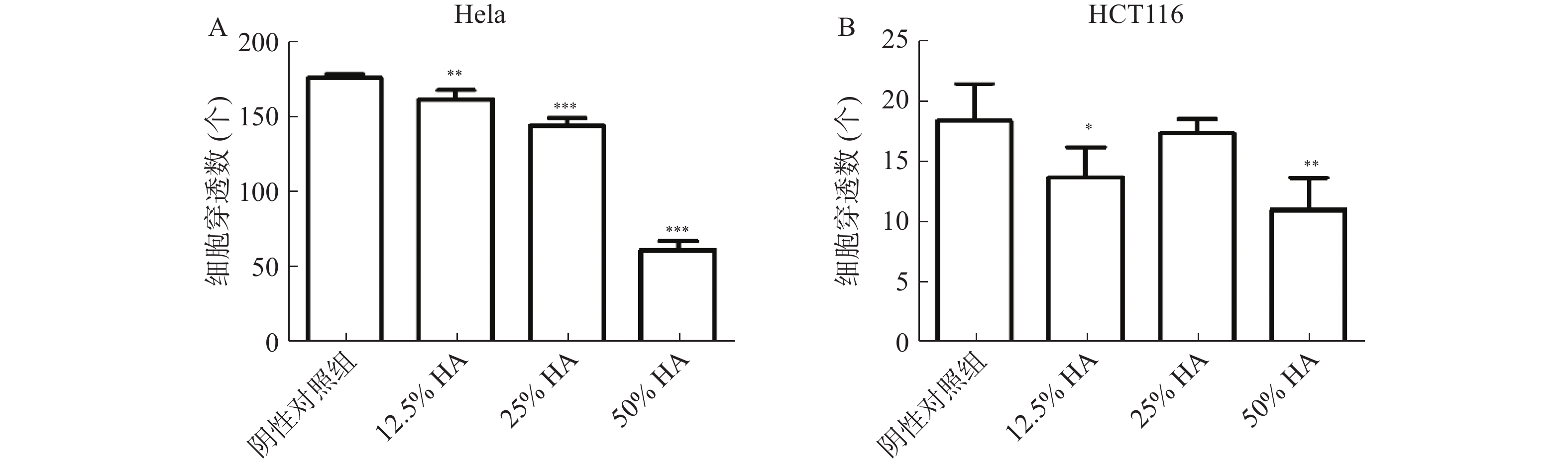
将状态良好的肿瘤细胞胰酶消化、离心后,分别用对应空白培养液重悬成单个肿瘤细胞悬液,显微镜下计数,然后分别稀释成以下浓度:Hela(1.5×105 个/ml),HCT116(5×105个/ml)。分别将肿瘤细胞混悬液和HA混匀,配成50%HA+50%肿瘤细胞悬液、25%HA+50%肿瘤细胞悬液+25%空白培养液、12.5%HA+50%肿瘤细胞悬液+37.5%空白培养液、50%肿瘤细胞悬液+50%空白培养液。将肿瘤细胞和HA混合液加入Transwell上层小室中,每个小室200 μl,Transwell下层腔室分别加入含10% FBS 对应培养液 600 μl。将Transwell培养板放入培养箱,培养24 h。取出Transwell小室,轻轻吸弃小室内培养液,用PBS洗1遍,甲醇固定15 min。用棉签轻轻擦去小室内层细胞,PBS洗2遍,加入结晶紫(0.1%)染色,室温放置20 min,PBS洗3遍。显微镜10倍物镜下观察、拍照和记数。
-
PBS重悬HCT116细胞,调浓度至107个/ml,将细胞接种于裸鼠皮下,每只动物0.2 ml,共12只,作为供瘤体。视肿瘤生长情况将裸鼠处死,取出瘤块,选取瘤块实质性部分,用生理盐水冲洗干净,剪成约1 mm×1 mm×1 mm 瘤块备用。
实验动物腹腔麻醉,取腹部正中切口,逐层开腹,找到盲肠位置。假手术组:将盲肠拉出后放回腹腔,逐层缝合伤口。其余组:轻轻划破结肠浆膜,用生物胶将瘤块固定在浆膜破损处。模型组和阳性对照组在瘤块接种处涂抹生理盐水0.07 ml,HA组涂抹HA 0.02 ml。逐层缝合伤口,观察记录裸鼠状态,5-Fu组腹腔注5-Fu 20 mg/kg,隔天给药,持续3周。
瘤块接种4周后将裸鼠处死,打开腹腔,用游标卡尺测量瘤块的长短径,按公式(V=ab2/2,a:瘤体最长直径;b:瘤体最短直径)计算体积,剥离瘤块,瘤块称重。
-
建立裸鼠结肠原位种植结肠癌CT26模型,方法同“2.3”项。瘤块接种4 d后,5-Fu组腹腔注射氟尿嘧啶注射液50 mg/kg,隔3 d给药一次,共4次。
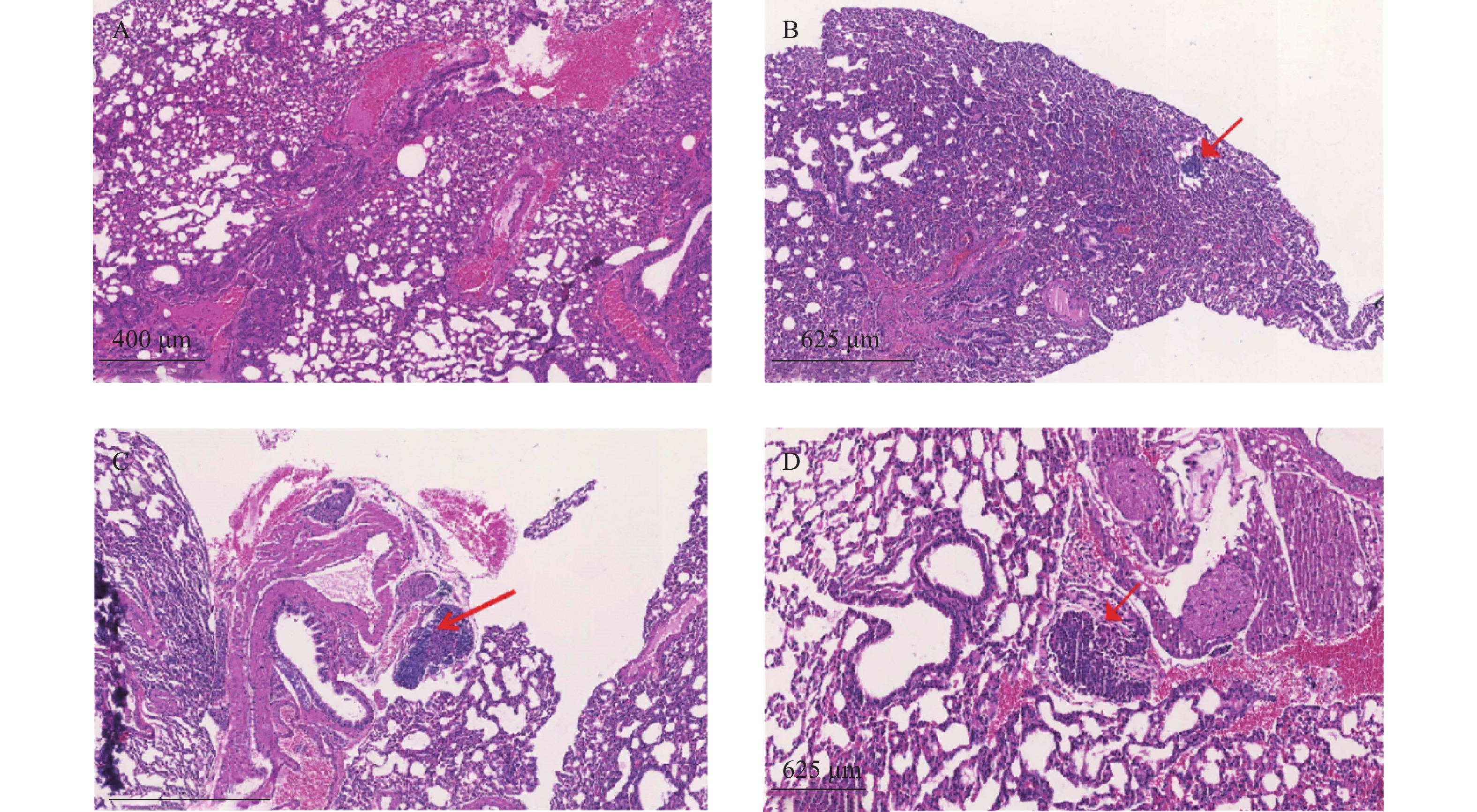
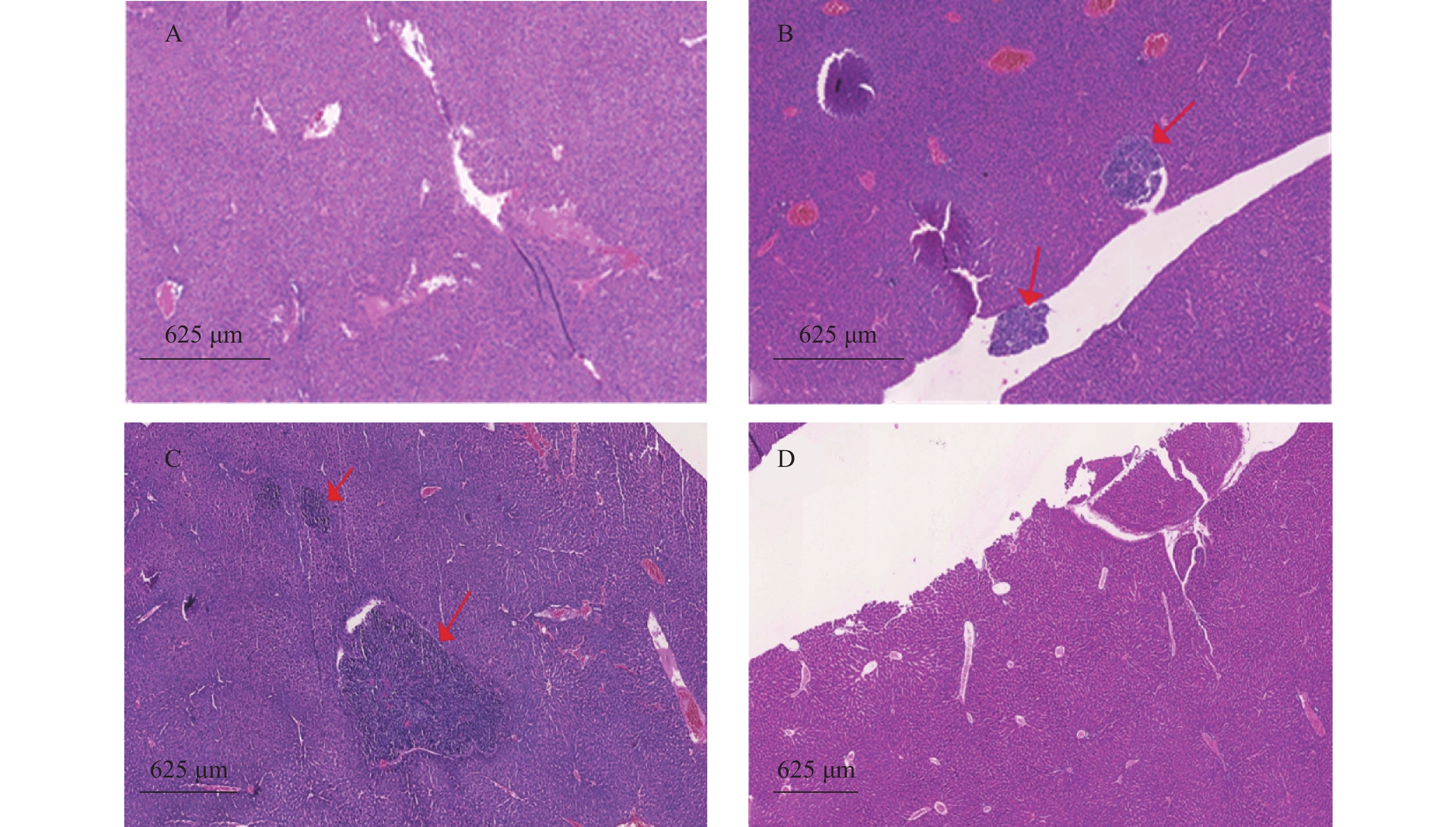
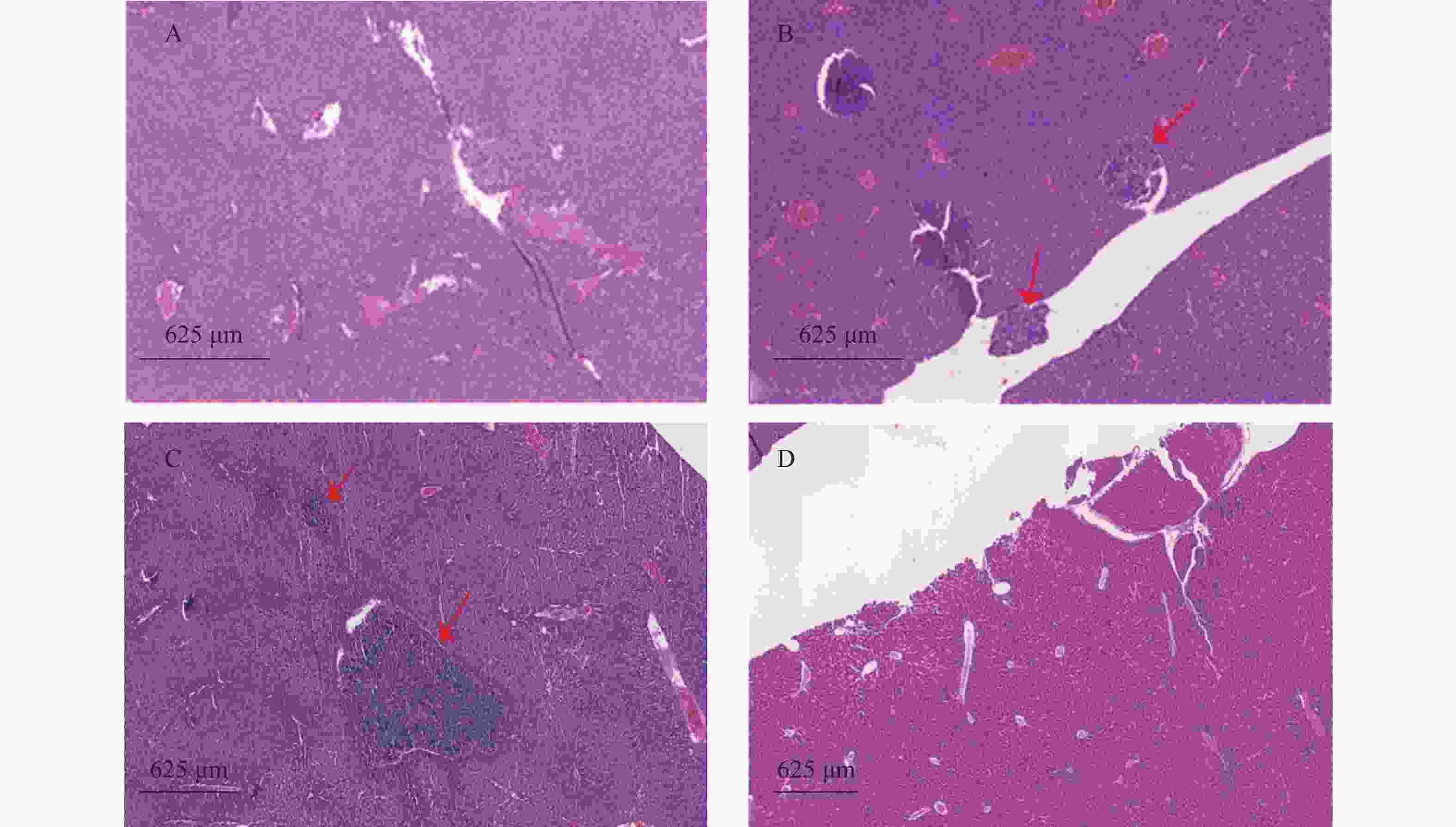
瘤块接种3周后将裸鼠脱颈处死,打开腹腔,观察肿瘤转移情况,取肺和肝脏,4%多聚甲醛固定、切片、HE染色,统计肿瘤转移率和转移病灶数。
-
实验数据用(
${\overline x}\pm s $ )表示,应用SPSS 21.0软件,数据进行正态性检验。符合正态分布采用one-way ANOVA统计方法进行显著性检验;不符合正态分布采用Friedman M 统计方法进行显著性检验。肿瘤转移率采用卡方检验。以P<0.05为差异有统计学意义。
2.1. MTT法检测肿瘤细胞的生长
2.2. Transwell检测肿瘤细胞的迁移
2.3. 原位种植瘤法考察裸鼠体内肿瘤的生长
2.4. 原位种植瘤法考察裸鼠体内肿瘤的转移
2.5. 统计方法
-
在体外实验中,肿瘤细胞Hela、HCT116和CT26在100% HA中均不能生长(图2)。Hela细胞在培养3 d和5 d时不同含量HA均不影响细胞的生长,7 d时25%HA和50%HA细胞生长率低于阴性对照组(P<0.001)。HCT116细胞培养7 d时,50%HA细胞生长率低于阴性对照组(P<0.01)。CT26细胞培养3 d时12.5%HA、25% HA和50%HA细胞生长率低于阴性对照组(P<0.001);培养5 d和7 d时,50%HA细胞生长率低于阴性对照组(P<0.001)。不同浓度5-Fu,培养3 d、5 d和7 d均抑制肿瘤细胞Hela、HCT116和CT26的生长,呈剂量相关性。
-
Transwell实验结果显示,高浓度HA体外抑制肿瘤细胞迁移。50%HA、25%HA和12.5%HA组Hela细胞穿透数均低于阴性对照组(P<0.01),HA含量越高,Hela细胞穿透数越少;50%HA和12.5%HA组HCT116细胞穿透数均低于阴性对照组(P<0.05),25%HA组HCT116细胞穿透数低于阴性对照组,无统计学差异(图3)。
-
裸鼠结肠原位接种HCT116瘤块后,HA组与模型组成瘤率均为100%,结果无差异。模型组裸鼠的瘤体体积达(339.1±258.0)mm3,5-Fu组裸鼠的瘤体体积为(128.5±111.9)mm3,小于模型组(P<0.01),HA组裸鼠的瘤体体积为(293.2±190.6)mm3,与模型组比较,差异无统计学意义;模型组裸鼠的瘤重为(0.359±0.219)g,5-Fu组裸鼠的瘤重为(0.149±0.105)g,小于模型组(P<0.001),HA组裸鼠的瘤重为(0.330±0.187)g,与模型组比较,差异无统计学意义。使用一定剂量的HA在裸鼠体内不影响HCT116的成瘤率,同时也不影响HCT116肿瘤的生长(表1)。
组别 动物数(只) 成瘤数(只) 成瘤率(%) 瘤体积(V/mm3) 瘤重(m/g) 假手术组 16 0 0 0.0 ± 0.0 0.000 ± 0.000 模型组 16 16 100 339.1 ± 258.0 0.359 ± 0.219 HA组 16 16 100 293.2 ± 190.6 0.330 ± 0.187 5-Fu组 16 14 87.5 128.5 ± 111.9** 0.149 ± 0.105*** **P<0.01、***P<0.001,与模型组比较 -
裸鼠结肠原位接种CT26瘤块,观察16 d后出现动物死亡,第18天处死存活裸鼠。模型组存活率为62.5%,HA组存活率68.8%,5-Fu组存活率100%,体内使用一定剂量的HA不影响裸鼠的存活率。模型组肝脏转移率90%,平均病灶数(2.2±1.7);5-Fu组肝脏转移率0.0%,低于模型组(P<0.001);平均病灶数(0.0±0.0),低于模型组(P<0.001)。HA组肝脏转移率36.4%,低于模型组(P<0.05);平均病灶数(0.6±1.0),低于模型组(P<0.01)。模型组肺转移率为40%,平均病灶数(0.5±0.7);5-Fu肺转移率6.3%,低于模型组(P<0.05);平均病灶数(0.1±0.3),低于模型组(P<0.05)。HA组肺转移率为18.2%,平均病灶数(0.2±0.4),与模型组均无统计学差异,见表2、图4和图5。
组别 动物数(只) 成活率(%) 肝转移 肺转移 转移率(%) 病灶数 转移率(%) 病灶数 假手术组 15 100.0 0.0 0.0 ± 0.0 0.0 0.0 ± 0.0 模型组 16 62.5 90.0 2.2 ± 1.7 40.0 0.5 ± 0.7 HA 组 16 68.8 36.4* 0.6 ± 1.0** 18.2 0.2 ± 0.4 5-Fu 16 100.0 0.0*** 0.0 ± 0.0*** 6.3* 0.1 ± 0.3* *P<0.05、**P<0.01、***P<0.001,与模型组比较
3.1. HA体外对肿瘤细胞生长的影响
3.2. HA体外对肿瘤细胞迁移的影响
3.3. HA体内对肿瘤细胞生长的影响
3.4. HA体内对肿瘤细胞转移的影响
-
很多胚胎迁移和增殖速率与胚胎组织中高浓度的透明质酸有关,而肿瘤组织和胚胎发育很相似,几十年前已经提出透明质酸可能对肿瘤生长很重要[7-9]。然而,本研究的HA为外源性高分子量HA,结果与内源性透明质酸结果不一致。在体外实验中,Hela、CT26和HCT116的细胞无法在100% HA中生长,表明HA不能作为肿瘤细胞培养基。此外,细胞生长速率随着HA的增加而不同程度的降低,说明高浓度HA可以抑制肿瘤生长,在低浓度时虽然没有明显的抑制作用,但也未促进肿瘤的增殖。体内结果证明,外源性HA对肿瘤细胞的生长无促进作用。
内源性低分子质量透明质酸(low molecular weight hyaluronan,LMWHA)可诱导细胞运动[10-11],促进血管生成,增加炎症免疫刺激和诱导高尔基体应激的能力[12],从而能促进肿瘤的迁移。而Aikaterini等[13-14]研究显示,透明质酸酶2(Hyal-2)低表达同时透明质酸合成酶(HAS1和HAS2)表达增高,导致产生高分子量透明质酸(high molecular weight hyaluronan,HMWHA)并在HT1080细胞的周围基质中沉积蓄积,结果HT1080细胞迁移能力显著降低,同时外源性HMWHA显著抑制HT1080细胞的迁移,当加入透明质酸酶后,HT1080细胞运动性显著增加。外源性透明质酸会促进透明质酸合成酶高表达[15],促进内源性HA合成作用,使机体合成HMWHA。本研究建立裸鼠原位种植瘤转移模型,结果HA组结肠癌的肝转移率在体内低于模型组(P<0.05)。HA在体内能抑制肿瘤的转移,可能与外源性HA能促使机体合成HMWHA有关。
本研究的主要目的是探讨HA是否会对腹部和盆腔肿瘤患者产生不良影响。结果表明,外源性HA未见对上述肿瘤的增殖有促进作用,在转移模型中HA表现出一定的抑制肿瘤迁移的作用,符合HA在肿瘤患者中应用的要求,但其机制还需进一步研究。











 DownLoad:
DownLoad: