-
复方首乌藤合剂是我院研制的特色制剂,主要由首乌藤、合欢皮、菟丝子、五味子、续断、蔓荆子、川芎、煅牡蛎八味药材配制而成,方中首乌藤为君药,合欢皮为臣药,菟丝子、五味子、续断为佐药,川芎、蔓荆子、煅牡蛎为使药,诸药合用,具有养心安神解郁、滋阴养肝、行血活气之功效,临床应用于神经衰弱性失眠、眩晕及脑外伤引起的头痛、头晕等症[1]。目前,该制剂的质量标准中主要控制其总黄酮、二苯乙烯苷含量[2],未包含多糖含量控制,相关研究表明,多糖具有预防、改善、治疗失眠的作用,且滋阴养肝效果明显[3-6]。因此,探究复方首乌藤合剂总多糖含量及多糖组成很有必要,本文在前期研究的基础上 对该制剂粗多糖的组成及总多糖含量进行了分析,现报道如下。
HTML
-
复方首乌藤合剂为本院自制(规格:40 ml,批号:201906132,201906133,201906211,20190624);D-甘露糖(批号:140651-201504,质量分数99.4%)、D-盐酸氨基葡萄糖(批号:140649-201606,质量分数100%)、半乳糖(批号:160226-201506,质量分数100%)、D-葡萄糖(批号:110833-201707,质量分数99.9%)、D-半乳糖醛酸(批号:111646-201702,质量分数95.1%),L-阿拉伯糖(批号:111506-200202,质量分数100%)、1-苯基-3-甲基-5-吡唑啉酮(PMP)、三氟乙酸(TFA)均购自中国食品药品检定研究院;氢氧化钠、盐酸、甲醇、三氯甲烷、正丁醇、浓硫酸、苯酚、无水乙醇均为分析纯; 水为本院自制纯化水。
-
紫外可见分光光度计(UNIC UV-2102 PCS,日本岛津);Agilent 1200高效液相色谱系统(DAD检测器,美国Agilent公司);电子分析天平(AUX220,日本岛津);磁力加热搅拌器(KQY78-1,金坛市科析仪器有限公司);数显恒温水浴锅(HH系列,金坛市科析仪器有限公司);电动离心机(LD5-2A,北京医用离心机厂);电热鼓风干燥箱(101A-1E,上海实验仪器有限公司);超声清洗器(KQ-500B,昆山市超声仪器有限公司);真空干燥箱(DZF-6050,上海医用恒温设备厂)。
1.1. 材料
1.2. 仪器
-
精密吸取25 ml复方首乌藤合剂,加入氯仿-正丁醇混合液(氯仿:正丁醇=5:1)20 ml,在磁力搅拌器上搅拌30 min,离心20 min(50 Hz,4000 r/min),回收上清液,去除蛋白质及有机溶剂;乙醇沉淀:向上清液中加入3倍体积的95%的乙醇,保持乙醇浓度在70%以上,静置过夜。离心15 min,收集沉淀物,80 ℃真空干燥3 h,得到相应批号的复方首乌藤合剂多糖粉末。
-
精密称取105 ℃干燥至恒重的D-无水葡萄糖0.1 g,置于100 ml容量瓶中,加水定容至刻度,备用。
-
精密称取复方首乌藤合剂多糖粉末0.1 g,置于250 ml容量瓶中,加水定容至刻度,得供试品原液;再精密吸取3 ml供试品原液至10 ml容量瓶中,加水定容,即为供试品溶液。
-
精密吸取对照品储备液6.0 ml,置于100 ml量瓶中,加水定容至刻度,即得对照品溶液。精密吸取对照品溶液1.0 ml,置于10 ml量瓶中,加入4%的苯酚溶液1.0 ml,振荡摇匀,然后加入7 ml浓硫酸,立即摇匀,100 ℃水浴15 min,然后冰浴15 min,冷却至室温。以相应试剂为空白,在400~550 nm范围内扫描光谱。结果发现,对照品溶液在486nm处有最大吸收,故选择486 nm。
-
精密吸取葡萄糖对照品储备液0、0.2、0.4、0.6、0.8、1.0 ml,分别置10 ml量瓶中,用水定容至刻度。然后各精密吸取1.0 ml,分别置于10 ml量瓶中,加入4%的苯酚溶液1.0 ml,振荡摇匀,然后加入7 ml浓硫酸,立即摇匀,100 ℃水浴15 min,然后冰浴15 min,冷却至室温,在486 nm波长处测定吸光度(Y)。以Y为纵坐标,浓度(X)为横坐标,绘制标准曲线,得到标准曲线方程:Y= 0.007X + 0.0105,r = 0.9982。
-
精密量取“2.2.1”项下的对照品溶液1.0 ml,按“2.3.1”项下方法测定吸光度,连续测定6次,根据标准曲线方程计算多糖含量,求RSD。结果得到RSD为0.24% (n=6),表明仪器精密度良好。
-
精密量取供试品溶液(批号:20190624)1.0 ml,置10 ml量瓶中,按“2.3.1”项下方法测定吸光度。在显色后0、10、20、40、60、80、100、120 min分别测定吸光度,求RSD。结果得到RSD为3.54% (n=7),表明该制剂多糖提取液在显色后120 min内稳定性良好。
-
取复方首乌藤合剂(批号:20190624)6份,按“2.2.2”项下方法制备供试品溶液,按“2.3.1”项下方法测定吸光度,计算多糖含量。结果测得6份供试品溶液中多糖的平均含量为18.23 mg/ml,RSD为0.33%(n=6),表明该方法重复性良好。
-
精密量取已知浓度为61 μg/ml的供试品溶液(批号:20190624)6份,每份0.5 ml,各加入0.5 ml浓度为63 μg/ml的葡萄糖对照品溶液,按“2.3.1”项下方法操作,于486 nm波长处测定吸光度,计算加样回收率。结果显示该方法的平均加样回收率为100.45%,RSD为1.57% (见表1)。
样品加入量 (μg) 葡萄糖加入量 (μg) 吸光度 测定量 (μg) 回收率 (%) 平均回收率 (%) RSD (%) 30.50 31.50 0.445 62.00 100.00 100.45 1.57 30.50 31.50 0.442 61.67 99.46 30.50 31.50 0.457 63.33 102.15 30.50 31.50 0.456 63.67 102.69 30.50 31.50 0.441 61.33 98.92 30.50 31.50 0.453 61.67 99.46 -
按“2.2.2”项下方法制备供试品溶液,按“2.3.1”项下方法测定吸光度,计算多糖含量。用外标法计算总多糖含量(以葡萄糖计),结果见表2。
批号 总多糖含量/(mg/ml) 平均值/(mg/ml) RSD/(%) 201906211 14.24 14.24 0.25 201906211 14.20 201906211 14.27 201906132 21.09 21.09 0.66 201906132 20.95 201906132 21.23 201906133 17.76 17.85 0.66 201906133 17.80 201906133 17.98 20190624 18.15 18.17 0.58 20190624 18.07 20190624 18.28 -
称取各单糖对照品: D-甘露糖、D-盐酸氨基葡萄糖、D-半乳糖醛酸、D-无水葡萄糖、半乳糖、L-阿拉伯糖等各约10 mg,精密称定,分别置于5 ml 量瓶中,加水至刻度,摇匀,即得。
-
取多糖粉末(批号:20190624)150 mg,精密称定,置于10 ml 具塞试管中,加水 3 ml,再加TFA3 ml,封管于110 ℃烘箱中水解1 h,取出,放冷,水浴蒸干,用甲醇1 ml溶解,蒸干,重复处理 3 次以除去残留的 TFA,残渣加水溶解[9],转移至 5 ml量瓶中,放冷,加水至刻度,摇匀,即得。
-
取上述 6种单糖对照品溶液各50 μl置于同一10 ml EP管中,供试品溶液1.0 ml置于10 ml EP管中,分别加入 1.0 ml 0.5 mol/L的PMP/甲醇溶液和1.0 ml 0.3 mol/L的氢氧化钠溶液,混合均匀,70 ℃水浴50 min,取出后冷却至室温,分别加入1.0 ml 0.3 mol/L的盐酸溶液中和,再加入2 ml氯仿萃取,取上清液,重复处理 3 次,除去未反应的PMP,0. 22 μm 滤膜滤过[9],即得。
-
色谱柱:Agilent Exted-C18 (4.6 mm×250 mm,5 μm);柱温:30 ℃;流动相:乙腈(A) - 0.1 mol/L的磷酸盐缓冲液(pH =6.8)(B)等度洗脱(A:B=16:84);流速:1.0 ml/min;检测波长250 nm;进样量:20 μl。
-
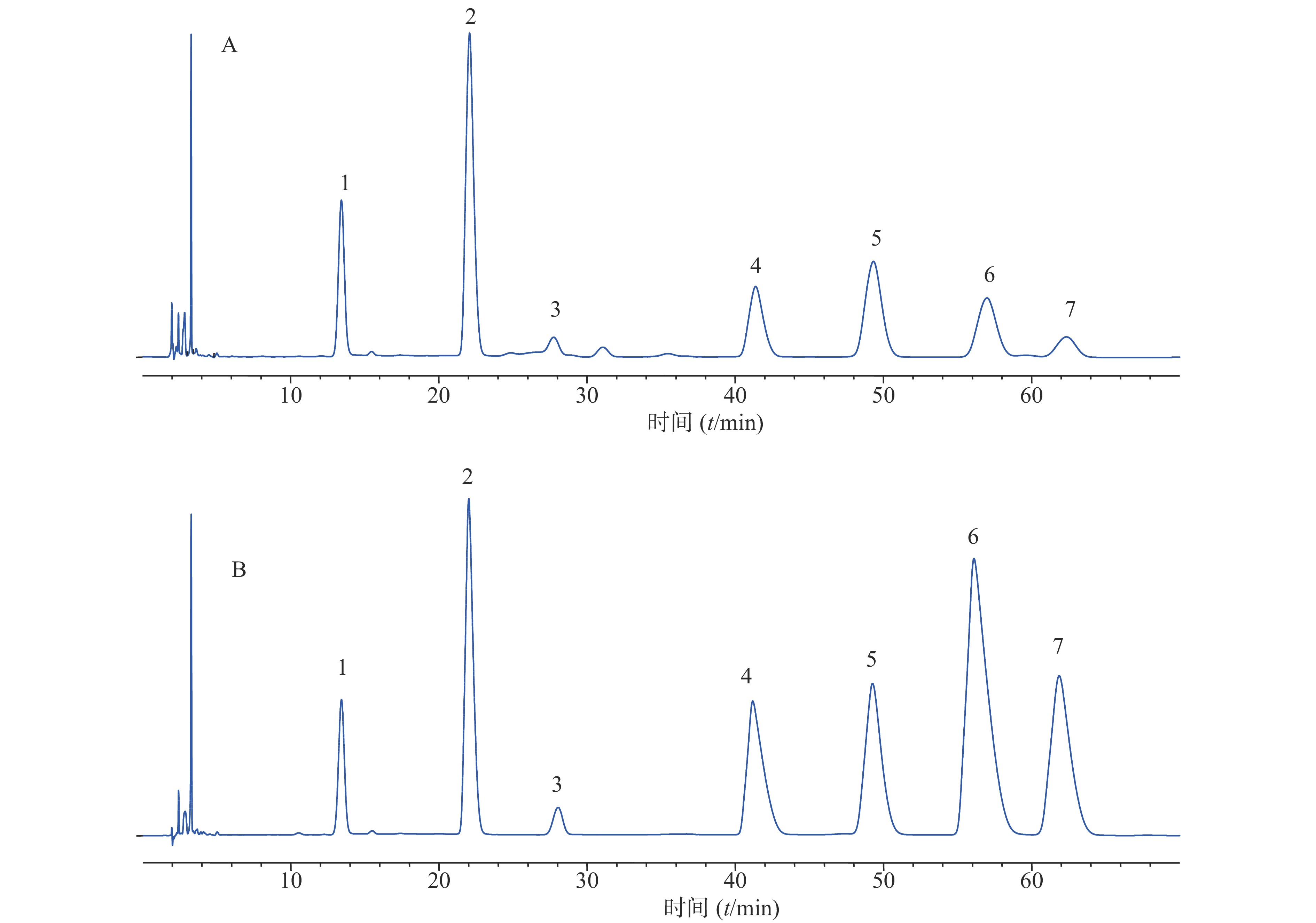
精密吸取适量“2.5.2”项下供试品溶液及“2.5.1”项下对照品溶液,按照“2.5.3”项下衍生方法进行衍生,按照“2.5.4”项下色谱条件进样,分别得到供试品溶液色谱图(图1),从复方首乌藤合剂多糖中识别出甘露糖、盐酸氨基葡萄糖、半乳糖醛酸、葡萄糖、半乳糖、阿拉伯糖等6 种单糖。
根据5次各单糖的峰面积比测定结果进行计算,甘露糖:盐酸氨基葡萄糖:半乳糖醛酸:葡萄糖:半乳糖:阿拉伯糖=9.10:0.26:1.00:3.02:4.14:2.12,可见甘露糖的峰面积占比接近50%,提示甘露糖可能是复方首乌藤合剂发挥特殊药效的原因之一。
2.1. 提取多糖组分
2.2. 总多糖含量的测定[7-8]
2.2.1. 对照品溶液配制
2.2.2. 供试品溶液配制
2.2.3. 最大吸收波长测定
2.3. 方法学考察
2.3.1. 标准曲线的制备及线性关系考察
2.3.2. 精密度试验
2.3.3. 稳定性试验
2.3.4. 重复性试验
2.3.5. 加样回收率试验
2.4. 总多糖含量测定
2.5. 单糖分析
2.5.1. 对照品溶液的制备
2.5.2. 供试品溶液的制备
2.5.3. 柱前衍生方法
2.5.4. 色谱条件
2.5.5. 结果
-
采用苯酚-硫酸法预实验时,发现供试品和对照品溶液如无水浴和冰浴处理,可能造成供试品过热且结果不稳定;若冰浴后无冷却至室温操作,吸光度结果偏小,故本实验选择先水浴,再冰浴,冷却至室温来避免以上情况出现。
-
在考察流动相时,发现乙腈比例大于25%时,各个色谱峰之间的分离度达不到要求;另外,如采用梯度洗脱方法,色谱峰6(半乳糖)与7(阿拉伯糖)之间分离度达不到要求,故选择等度洗脱。综合考虑,选择0.1 mol/L的磷酸盐缓冲液(pH=6.8)等度洗脱作为本实验流动相。
-
复方首乌藤合剂是由首乌藤、合欢皮、菟丝子、五味子、续断、蔓荆子、川芎、煅牡蛎八味药材配制而成的中药复方制剂,甘露糖的峰面积占比接近50%。根据相关研究发现,首乌藤中单糖成分以阿拉伯糖及半乳糖为主[10];合欢皮多糖较高,但未对单糖组分进行分析 [11];菟丝子多糖的主要组成是甘露糖,且含量最高[12];五味子以葡萄糖为主,甘露糖含量低[13];蔓荆子多糖含量较低[14];川芎多糖以葡萄糖及半乳糖为主[15]。综上可知,甘露糖可能主要来源于菟丝子,葡萄糖可能主要来源于五味子、川芎,阿拉伯糖可能主要来源于首乌藤。
3.1. 苯酚-硫酸法的样品处理
3.2. 单糖组分洗脱方法的选择
3.3. 制剂中总多糖含量及单糖组分的探讨
-
综上所述,采用苯酚硫酸法结合紫外分光光度法可以较为准确测定复方首乌藤合剂总多糖含量,柱前衍生化法结合高效液相色谱适用于分析该制剂多糖水解产物中的单糖组分,上述方法可为复方首乌藤合剂多糖质量控制提供参考。







 DownLoad:
DownLoad: