-
新型冠状病毒肺炎(COVID-19)由严重急性呼吸综合征冠状病毒2型(SARS-CoV-2)引起[1-2]。SARS-CoV-2是冠状病毒科和β冠状病毒属的成员,是影响人类呼吸功能的新冠肺炎大流行的病原体[1, 3-4],因其对公共卫生构成严重威胁而受到全球关注。中医对传染性疾病的认识较早,早在秦汉时期的《黄帝内经》中就记载有“五疫之至,皆相染易,无问大小,病状相似”。数年来,传统中医药在防病治病中积累了丰富的临床经验,开拓了数以万计的中药复方[5]。根据我国中医药出版社在2020年2月出版发行的《新型冠状病毒肺炎中医诊疗手册》,新冠肺炎属中医“瘟疫”范畴,通过分析历代中医药对瘟疫的防治经验,对于新冠肺炎的预防和治疗具有积极作用。
清肺排毒汤作为国家卫健委、国家中医药管理局推荐在新冠肺炎临床治疗期使用的中药复方,在此次疫情防控中起到关键性的作用,其由麻杏石甘汤、射干麻黄汤、小柴胡汤、五苓散、橘枳姜汤等加减化裁而成[6],方中各味药材在新冠肺炎不同病理过程治疗阶段都发挥重要作用[7-8]。中药复方麻杏石甘汤,始载于张仲景《伤寒杂病论》,书中所述“若汗出喘,无大热者,可与麻黄杏仁石膏甘草汤”。原方由麻黄、杏仁、石膏和甘草四味药配伍而成[9],君药为麻黄,辛苦宣肺、解表平喘;臣药为石膏,辛凉宣泄,君臣配合发散肺经郁热而平喘;佐药为杏仁,宣降肺气,辅助麻黄止咳平喘;使药为甘草,协调诸药[10],主治外感风邪、邪热壅肺证[4, 11-13]。多年以来,以麻杏石甘汤为基础方的各类制剂广泛应用于临床中。近年来,研究学者们在麻杏石甘汤的研究上也取得了众多成果,其具有多种药理活性,包括抗流感病毒活性,改善微血管通透性过高和抑制炎症反应等[14]。袁丽等[15]使用网络药理学初步预测了麻杏石甘汤对新冠肺炎的抗病毒和抗炎作用,并建立了IL-6诱导的大鼠肺上皮Ⅱ型细胞(RLE-6TN)损伤模型,探讨麻杏石甘汤的抗炎活性和可能的作用机制。吴佳等[16]探讨以麻杏石甘汤联合西药对症治疗新型冠状病毒肺炎的临床疗效及不良反应,研究结果表明,加味麻杏石甘汤联合推荐的治疗方案可促进新冠肺炎患者的康复,而且不增加不良反应的风险。
同化学药相比,中药研究最大的困难在于它是一个复杂体系。明确中药复方的化学成分对中药的药效与作用机制的阐明具有重要意义。本研究利用 UPLC-QTOF/MS技术,系统分析麻杏石甘汤的化学组分,其目的在于揭示麻杏石甘汤中各组方的药理活性与组方原理,为促进传统中医药方剂在新冠肺炎临床实践中提供重要的参考数据。
-
安捷伦1290型超高效液相色谱仪、6538 UHD Accurate-Mass Q-TOF/MS、MassHunter工作站和质谱分析软件 (美国Agilent公司);低温高速离心机、低温双开门冰箱(美国Thermo公司);循环水式真空泵(上海东玺制冷仪器设备有限公司);涡旋仪(美国Labnet公司);电子天平(日本A&D Company Limited 公司)。
-
麻黄、甘草、苦杏仁、石膏(上海雷允上医药公司) ;苦杏仁苷(29883-15-6,纯度≥98%)、甘草苷(551-15-5,纯度≥98%)、甘草酸(1405-86-3,纯度≥98%)购自成都格利普生物科技有限公司;麻黄碱(50-98-6,纯度≥98%,中国食品药品检定研究院);二甲基亚砜、乙腈、甲醇、甲酸(美国Sigma Chemical公司);屈臣氏纯净水(广州屈臣氏食品饮料有限公司)。
-
依照《伤寒杂病论》中麻杏石甘汤处方的配伍,用电子天平称取药材麻黄9 g,苦杏仁9 g,石膏18 g,甘草6 g放置于干净的1000 ml的圆底烧瓶内,加入纯化水1.4 L,先浸泡1 h,然后冷凝回流提取2 h。提取液用四层纱布过滤,得滤液。将滤渣再次用1.4 L纯化水回流提取1 h,四层纱布过滤,将两次滤液合并后,使用旋转蒸发仪浓缩至50 ml。麻杏石甘汤13 000×g离心10 min,然后,吸取上清液用0.22 μm微孔滤膜过滤,即可得到麻杏石甘汤供试品溶液,冰箱4 ℃冷藏,备用。分别准确称取苦杏仁苷,甘草苷,甘草酸,麻黄碱各适量,用二甲基亚砜溶剂进行溶解稀释, 定容, 即可得到1 mg/ml的单一成分对照品储备液;分别精密量取100 μl混合,二甲基亚砜定容,制成100 μg/ml的对照品混合母液,分装后4 ℃冰箱冷藏,备用。
-
色谱柱:UPLC BEH C18柱(2.1 mm×100 mm,2.5 μm);流动相A:含0.1%甲酸的水,流动相B:含0.1%甲酸的乙腈,梯度洗脱:A相为0~2 min,95%,2~13 min,95%~5%,13~15 min,5%,B相为0~2 min,5%,2~13 min,5%~95%,13~15 min,95%;进样体积:3 μl;样品进样前基线用流动相平衡5~10 min;流速:0.4 ml/min;柱温:40 ℃;检测时间15 min。
-
离子源:电喷雾(ESI)离子源;正负离子模式下采集数据;扫描模式为:全扫描/数据依赖的二级扫描(Full scan/ddMS2)。ESI源条件如下:干燥气温度:350 ℃,干燥气体流量:11 L/min;碎裂电压:120 V;毛细管电压:4000 V(ESI+)/3500 V(ESI-);质谱扫描范围:50~1500 m/z。正离子模式下参考离子m/z取值范围为121.0509 ~ 922.0098,负离子模式下参考离子m/z取值范围为112.9856 ~ 1033.9881。MS2采用40 eV的碰撞能量对母离子进行二级碎裂。得到的数据使用安捷伦MassHunter工作站进行计算和分析。
-
采用UPLC-QTOF-MS/MS技术对麻杏石甘汤化学成分的数据信息进行了定性分析,我们通过查阅国内外文献、数据库(HMDB, PubChem)等,建立麻杏石甘汤的化学成分数据库,利用QTOF/MS提供的精确分子量导入MassHunter分析软件,对采集的数据与化合物库进行进一步的比对和鉴定。
-
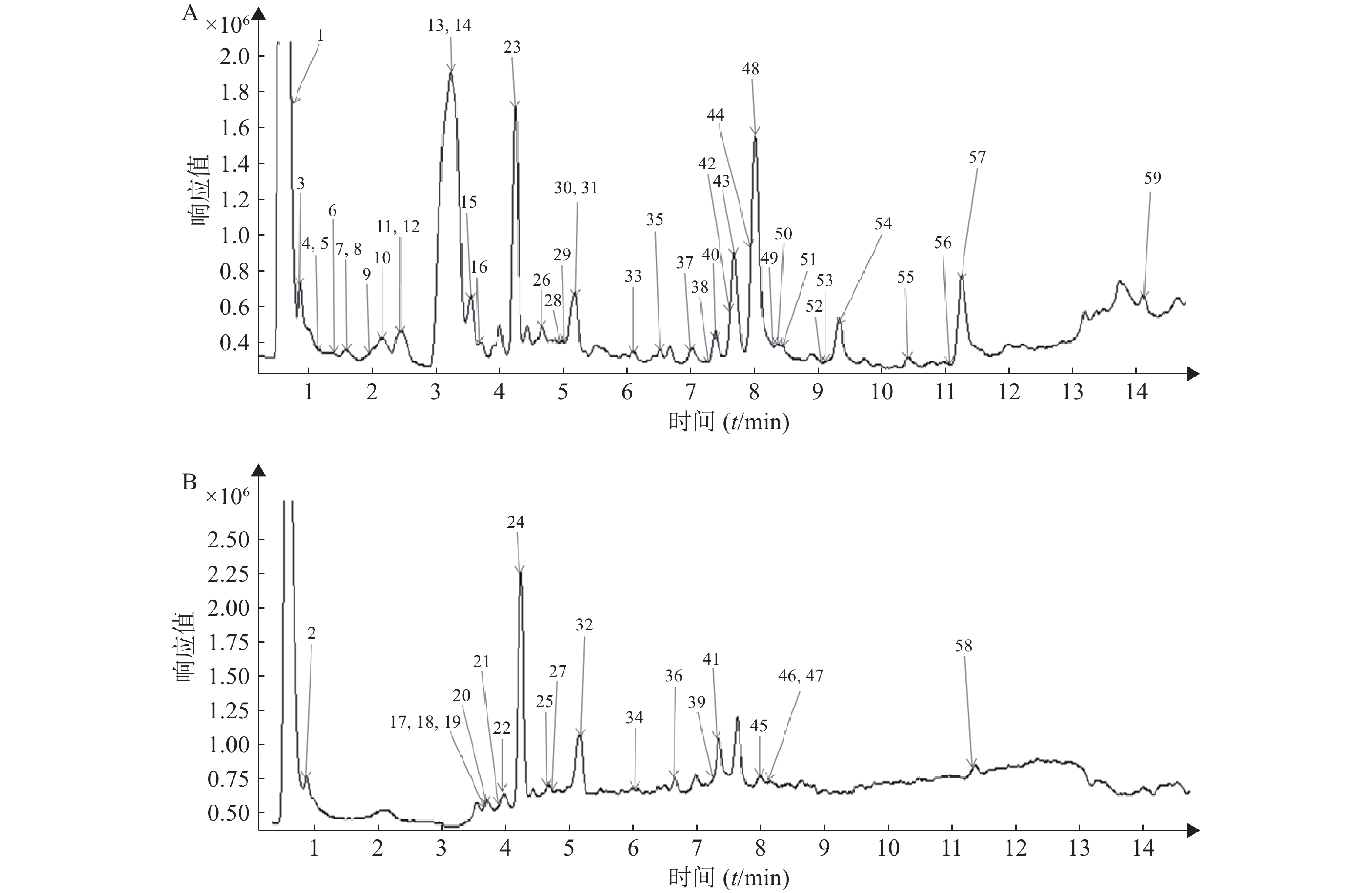
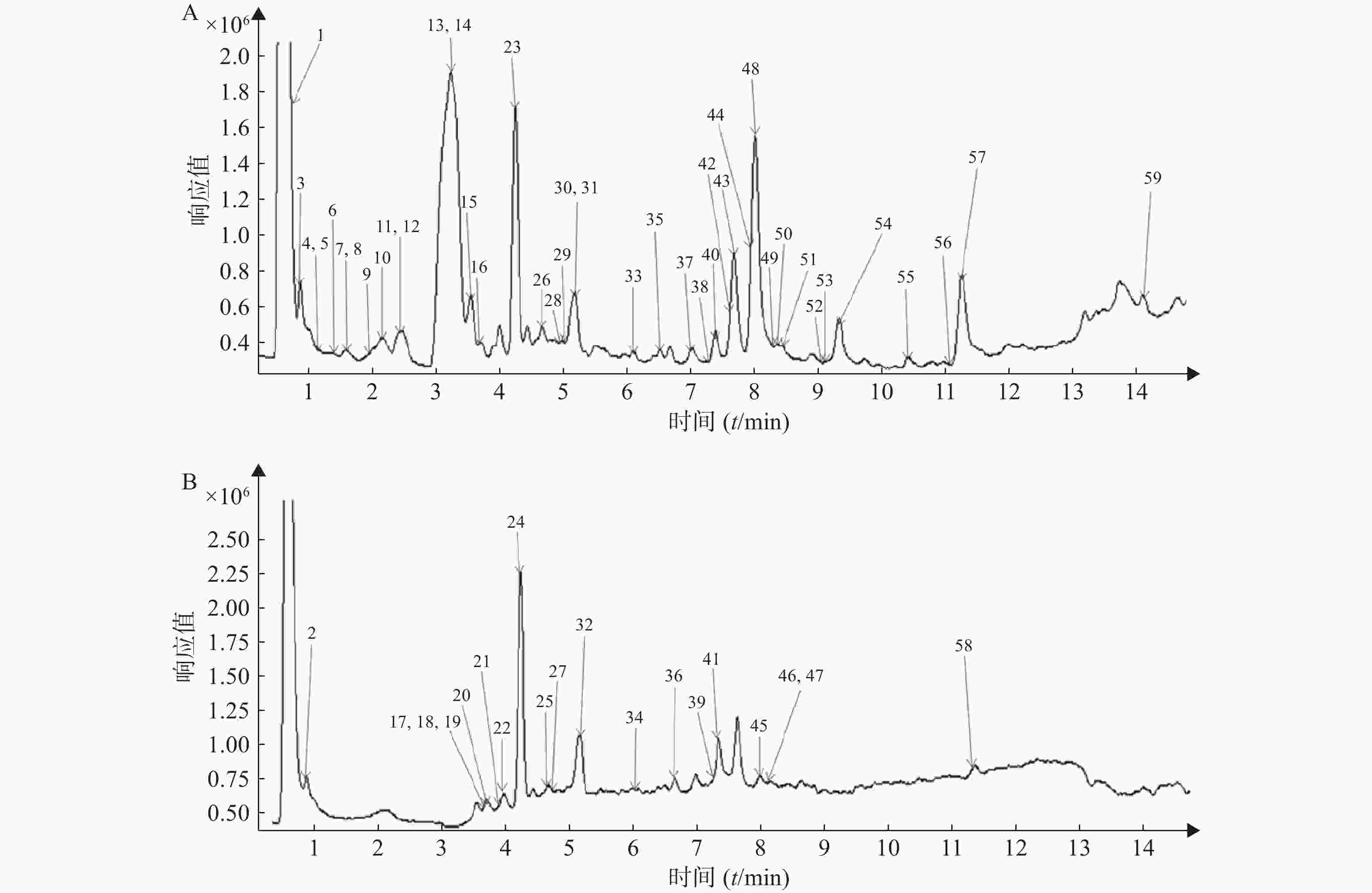
按照“2.1”和“2.2”项下色谱质谱条件,各种化合物在正、负离子扫描模式下的分离效果和离子化程度均比较明显,得到麻杏石甘汤供试品溶液正、负离子模式下的UPLC-QTOF/MS总离子流图(见图1),结合麻杏石甘汤的化合物自建库,初步筛选出59种化合物,其中正离子模式下有42种化合物,而负离子模式下则有30种化合物,同时在正离子模式下和负离子模式下鉴别出了13种化合物。
-
将所得数据导入MassHunter分析软件进行采集和分析,并且通过与化学成分数据库逐一比对得到麻杏石甘汤化合物成分的鉴定结果。共鉴定出59个化合物如表1所示,为进一步确定该复方的成分,本研究结合购买的对照品和国内外报道文献在120 V电压、40 eV条件下对所得数据中可能存在同分异构体的化合物 (甘草苷,甘草酸,麻黄碱,苦杏仁苷)进行了二级质谱碎片离子的分析与鉴定。
序号 保留时间(min) 化合物 分子式 离子模式 m/z 质量 误差(ppm) 药材来源 1 0.645
0.657蔗糖 C12H22O11 [M+Na]+
[M+HCOO]−365.1045
387.1176342.1163
342.1194−2.15
9.37苦杏仁 2 0.905 柠檬酸 C6H8O7 [M-H]− 191.0911 192.0271 0.54 苦杏仁 3 0.918 腺苷 C10H13N5O4 [M+H]+ 268.1024 267.0949 −6.79 苦杏仁 4 1.034 异亮氨酸 C6H13NO2 [M+H]+ 132.1012 131.0939 −5.88 苦杏仁 5 1.034 丙位己内酯 C6H10O2 [M+NH4]+ 132.1012 114.0673 −6.68 苦杏仁 6 1.572 复盆子酮 C10H12O2 [M+NH4]+ 182.1165 164.0824 −8 麻黄 7 1.630 1-苯基-1,2-丙二酮 C9H8O2 [M+NH4]+ 166.0857 148.0519 −3.55 麻黄 8 1.630 苯丙氨酸 C9H11NO2 [M+H]+ 166.0852 165.0784 −3.3 苦杏仁 9 2.118
2.155杏仁核酰胺 C20H29NO12 [M+Na]+
[M+HCOO]−498.1582
520.1677475.1686
475.1694−0.73
0.99苦杏仁 10 2.176 吲哚酮 C8H7NO [M+H]+ 134.0599 133.0525 −1.66 苦杏仁 11 2.457 间甲基丙酮 C9H10O [M+NH4]+ 152.1065 134.0747 −3.78 麻黄 12 2.457 去甲基麻黄碱 C9H13NO [M+H]+ 152.1065 151.0922 −3.54 麻黄 13 3.244 麻黄碱 C10H15NO [M+H]+ 166.1221 165.1448 −3.27 麻黄 14 3.244 4-异丙基-苯甲醛 C1OH12O [M+NH4]+ 166.1221 148.0883 −3.62 麻黄 15 3.542 O-N-甲基伪麻黄碱 C11H17NO [M+H]+ 180.1382 179.1308 −1.08 麻黄 16 3.583
3.603扁桃酸-β-龙胆二糖苷 C20H28O13 [M+Na]+
[M-H]−499.1420
475.1469476.1525
476.1542−1.02
2.45苦杏仁 17 3.727 对羟基苯甲酸乙酯 C9H10O3 [M-H]− 165.0555 166.0628 −1.3 麻黄 18 3.727 苯甲醛 C7H6O [M+CH3COO]− 165.0556 106.0417 −1.83 麻黄 19 3.727 苯乙酮 C8H8O [M+HCOO]− 165.0555 120.0573 −1.93 麻黄 20 3.752
3.804对香豆酸 C9H8O3 [M+HCOO]−
[M+H]+209.0450
165.0542164.0469
164.0464−2.43
−5.88麻黄 21 3.992
4.005(+)-儿茶素 C15H14O6 [M-H]−
[M+H]+289.0724
291.0850290.0793
290.07760.97
−4.94麻黄、苦杏仁 22 4.034 苄基-β-龙胆二酸 C19H28O11 [M+HCOO]− 477.1618 432.1632 0.09 苦杏仁 23 4.270
4.270
4.274苦杏仁苷 C20H27NO11 [M+H]+ [M+NH4]+
[M+HCOO]−458.1649
475.1927
502.1570457.1587
457.1587
457.1590−0.62
−0.62
−1.46苦杏仁 24 4.274 6″-O-乙酰苦参碱 C23H24O10 [M+CH3COO]− 519.1482 460.1341 −6.1 甘草 25 4.712 异夏佛塔苷 C26H28O14 [M-H]− 563.1422 564.1481 0.4 甘草 26 4.717 夏佛塔苷 C26H28O14 [M+H]+ 565.1552 564.1472 −1.25 甘草 27 4.754 野黑樱苷 C14H17NO6 [M+HCOO]− 340.1035 295.1051 −1.79 苦杏仁 28 5.007
5.0271-甲氧基槲皮素 C27H30O14 [M+H]+
[M-H]−579.1700
577.1563578.1630
578.1639−1.04
0.51甘草 29 5.147
5.176芹糖异甘草苷 C26H30O13 [M+H]+
[M-H]−551.1744
549.1619550.1672
550.1690−2.61
0.56甘草 30 5.205 4',7-二羟基黄酮 C15H12O4 [M+H]+ 257.0802 256.0726 −2.72 甘草 31 5.205
5.225甘草苷 C21H22O9 [M+H]+
[M-H]−419.1377
417.1203418.1267
418.12730.84
2.26甘草、苦杏仁 32 5.225 槲皮素 C20H20O7 [M+HCOO]− 417.1203 372.1220 2.83 甘草 33 6.132
6.161芒柄花苷 C22H22O9 [M+H]+
[M+HCOO]−431.1330
475.1249430.1252
430.1260−2.86
−1甘草 34 6.161 芹菜素-5-鼠李糖苷 C21H20O9 [M+CH3COO]− 475.1248 416.1105 −0.57 麻黄 35 6.687 甘草皂苷A3 C48H72O21 [M+H]+ 985.4643 984.4567 0.11 甘草 36 6.964 甘草皂苷E2 C42H60O16 [M+CH3COO]− 879.3996 820.3863 −2.28 甘草 37 7.026 甘草内酯 C30H44O4 [M+H]+ 469.3302 468.3230 −1.99 甘草 38 7.407
7.410田蓟苷 C22H22O10 [M+Na]+
[M+HCOO]−469.1100
491.1210446.1209
446.1223−0.88
2.19麻黄 39 7.410 芹菜甙元-8-O-葡萄糖苷 C21H20O10 [M+CH3COO]− 491.1211 432.1068 2.77 麻黄 40 7.415
7.419甘草皂苷G2 C42H62O17 [M+H]+
[M-H]−839.4060
837.3909838.3982
838.3980−0.6
−0.8甘草 41 7.419 光甘草轮 C16H10O6 [M+CH3COO]− 357.0631 298.0489 3.88 甘草 42 7.668 18-α-甘草次酸 C30H46O4 [M+H]+ 471.3464 470.3388 −1.72 甘草 43 7.697
7.717甘草酸 C42H62O16 [M+H]+
[M-H]−823.4107
821.3976822.4030
822.4044−0.93
0.78苦杏仁、
甘草44 8.044 反式-2-壬烯醛 C9H16O [M+NH4]+ 158.1536 140.1198 −6.07 麻黄 45 8.255 3-丁基-3a,4,5,6-四氢-顺式-1(3H)-异苯并呋喃酮 C12H18O2 [M+CH3COO]− 253.1441 194.1301 −2.92 麻黄 46 8.263 反式-2-癸烯酸乙酯 C12H22O2 [M+HCOO]− 243.1593 198.1617 −1.26 麻黄 47 8.263 2-十一烯酸 C11H20O2 [M+CH3COO]− 243.1597 184.1459 −2.32 苦杏仁 48 8.284 甘草皂苷J2 C42H64O16 [M+H]+ 825.4220 824.4146 −5.92 甘草 49 8.375 十六烷酸 C16H32O2 [M+NH4]+ 274.2734 256.2394 −3.33 麻黄 50 8.458 2-十四酮 C14H28O [M+NH4]+ 230.2467 212.2127 −6.07 甘草 51 8.483 2-羟基十六烷酸 C16H32O3 [M+NH4]+ 290.2674 272.2339 −4.56 苦杏仁 52 9.360 康唑烷酮 C26H28O6 [M+H]+ 437.1925 436.1852 −7.69 甘草 53 9.369 双(4-乙基亚苯亚甲基)山梨糖醇 C24H30O6 [M+H]+ 415.2111 414.2035 −1.76 苦杏仁 54 9.451 棕榈酸乙酯 C18H36O2 [M+NH4]+ 302.3040 284.2702 −4.79 麻黄 55 10.428 花生酸 C2OH4OO2 [M+NH4]+ 330.3354 312.3015 −4.23 麻黄 56 11.280 对苯二甲酸二丁酯 C16H22O4 [M+H]+ 279.1585 278.1509 −3.11 苦杏仁 57 11.314 二十二烷酸 C22H44O2 [M+NH4]+ 358.3663 340.3324 −5.08 苦杏仁 58 11.466 2,3,4-三甲基-5-苯基恶唑啉 C12H17NO [M+CH3COO]− 250.1457 191.1318 3.86 麻黄 59 14.310 苯乙烯 C8H8 [M+NH4]+ 122.0962 104.0621 −4.99 麻黄 -
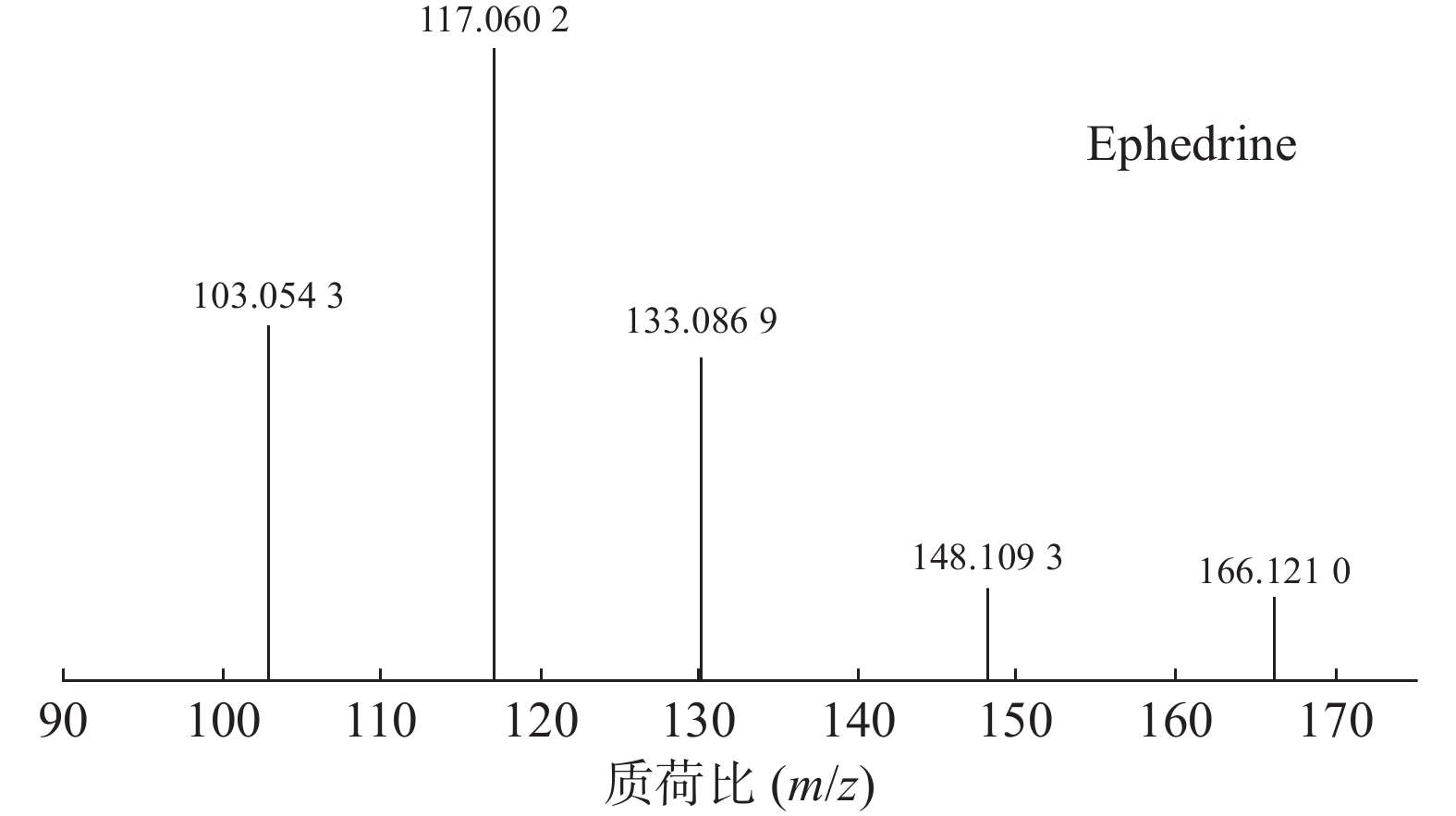
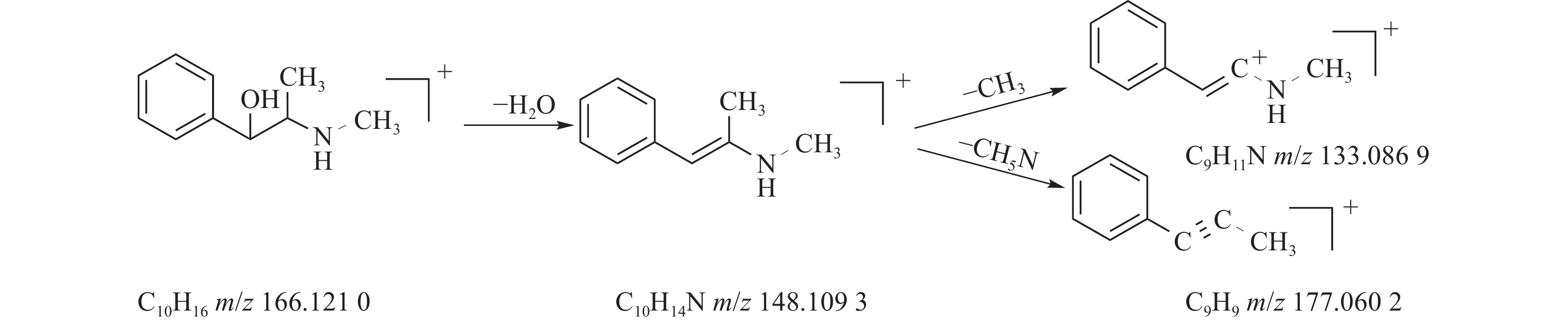
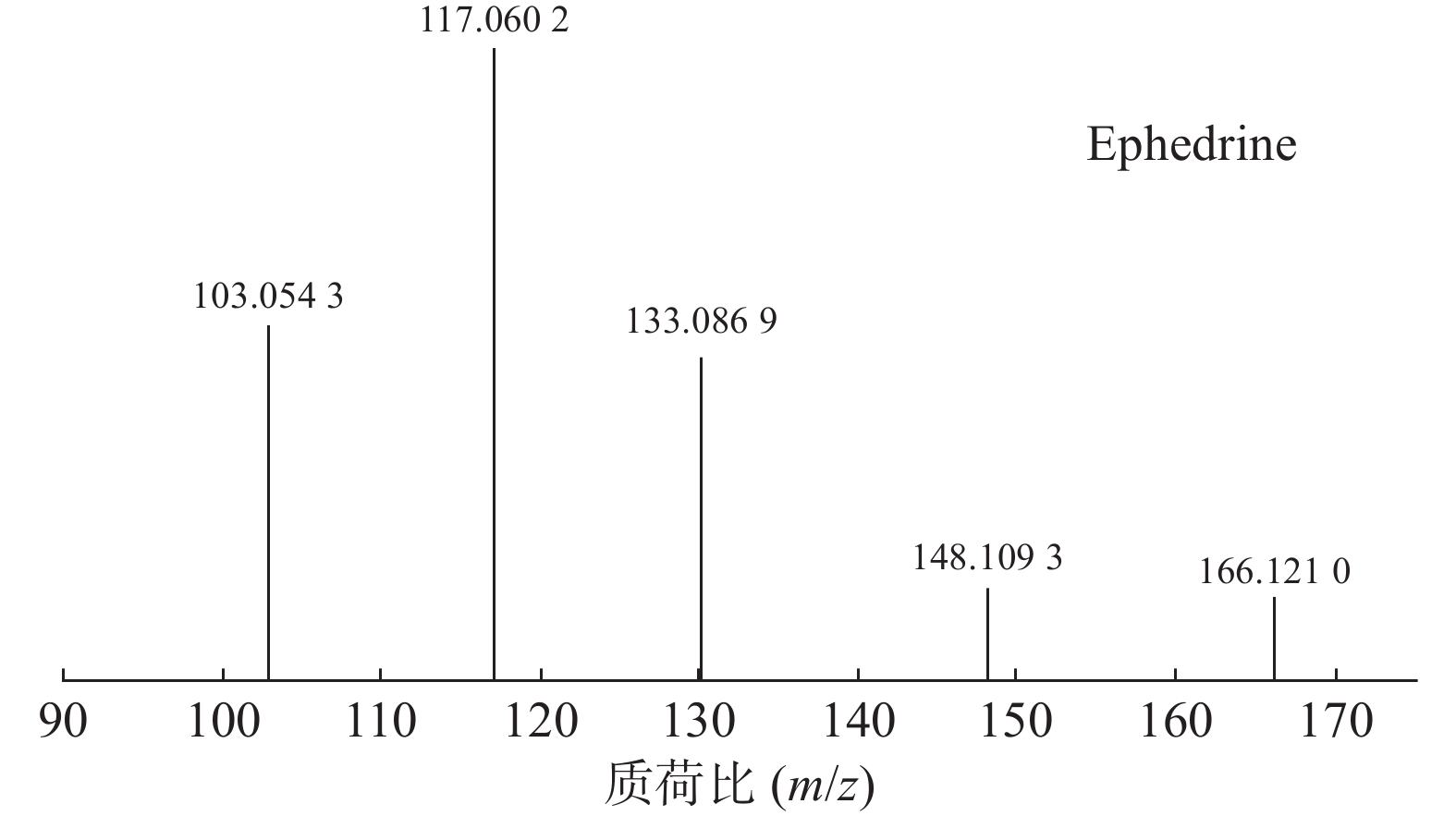
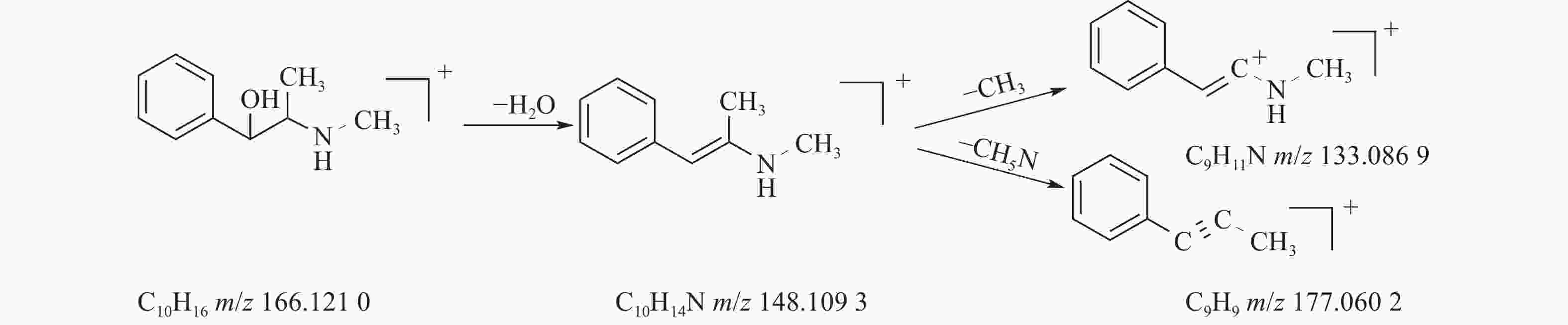
麻黄的主要有效成分为总生物碱,其具有平喘的功效,麻黄碱不仅是临床常用的生物碱类化合物,并且是麻黄中的标志性成分,其作用于支气管平滑肌并伴有解痉的疗效,以达到止咳平喘的效果。在正离子模式下,13号峰的母离子峰是m/z 166.1210[M+H]+ ,m/z 148.1093为该化合物的主要碎片离子峰,可判断为母离子峰先脱去一分子的H2O而得;得到的碎片离子脱掉一个CH3即可得到m/z 133.0869[M+H-H2O-CH3]+的碎片离子;碎片离子m/z148.1093[M+H-H2O]+进一步脱掉一个CH3NH2,得到碎片离子m/z 117.0602[M+H-H2O-CH5N]+,对照品的二级质谱碎片离子图,如图2所示,同时参考麻黄碱的碎片离子(m/z),根据生物碱类化合物的裂解规律可以推测出该峰为麻黄碱,裂解途径如图3所示。
-
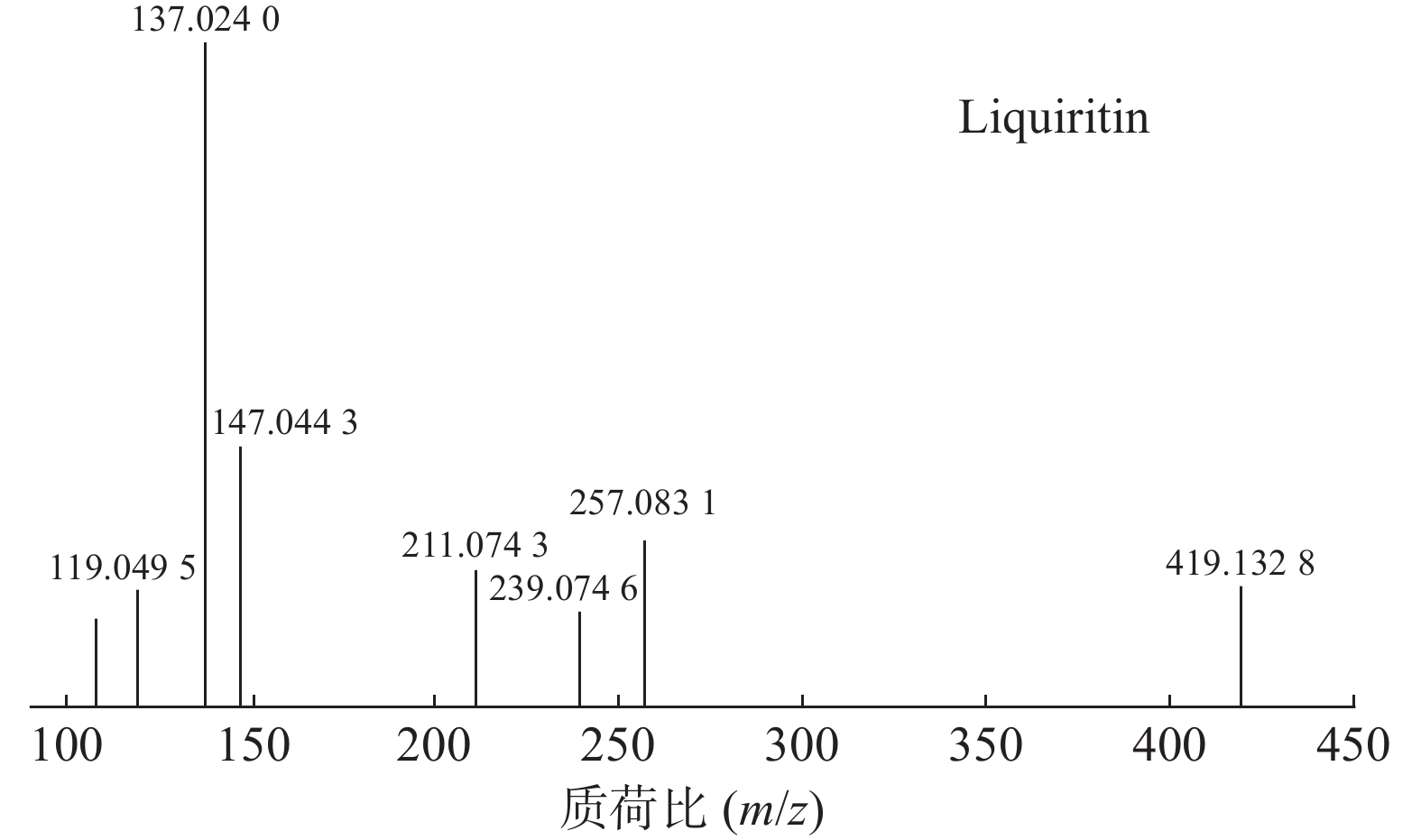
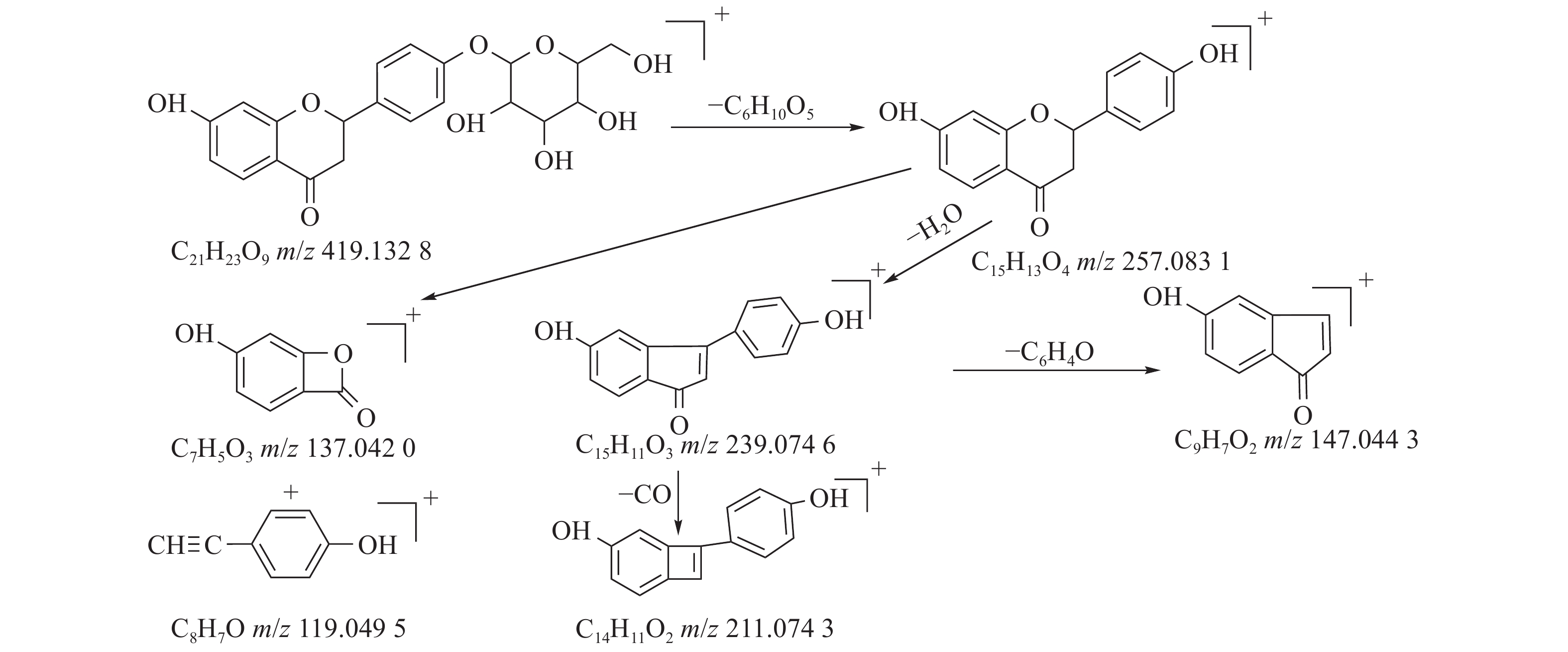
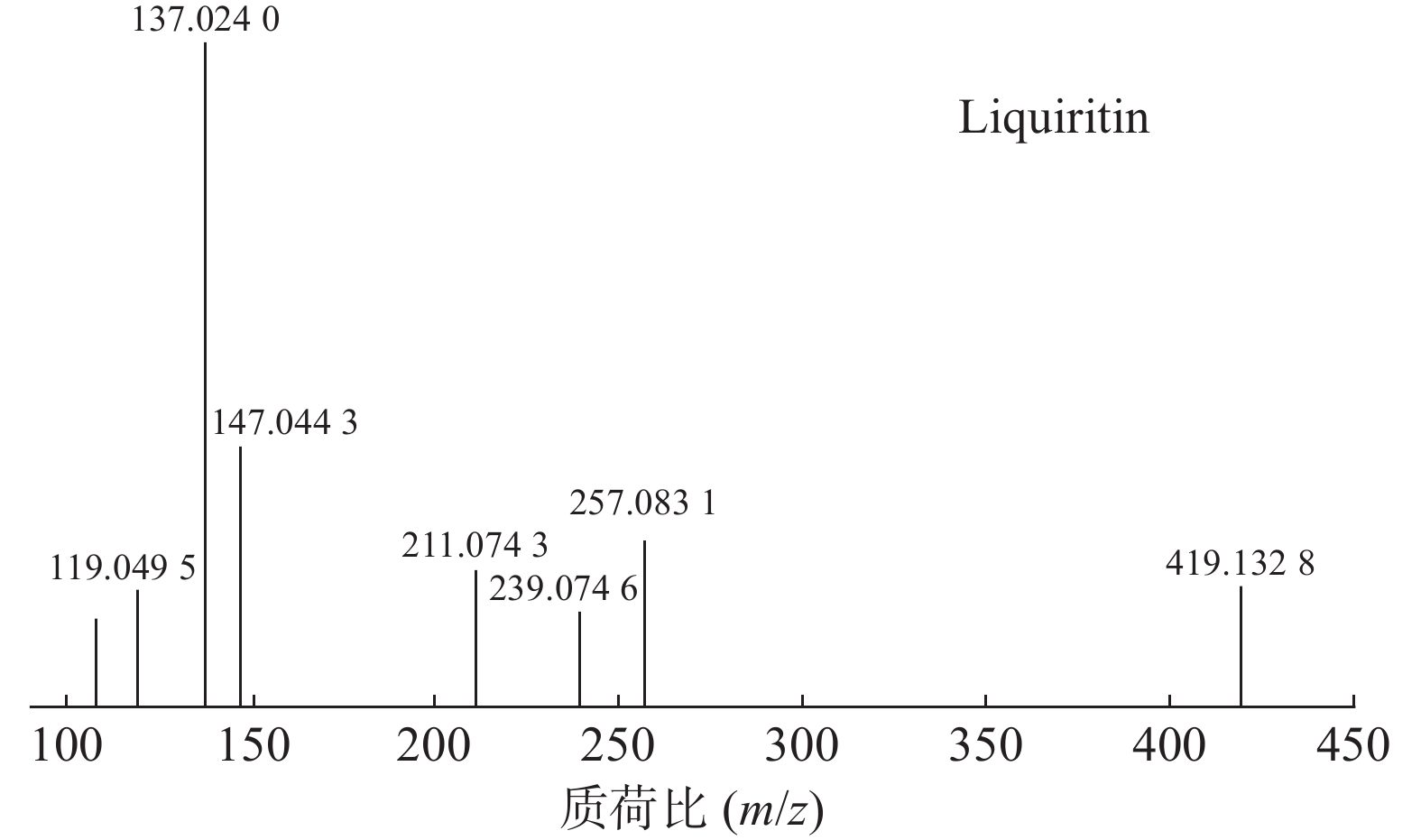
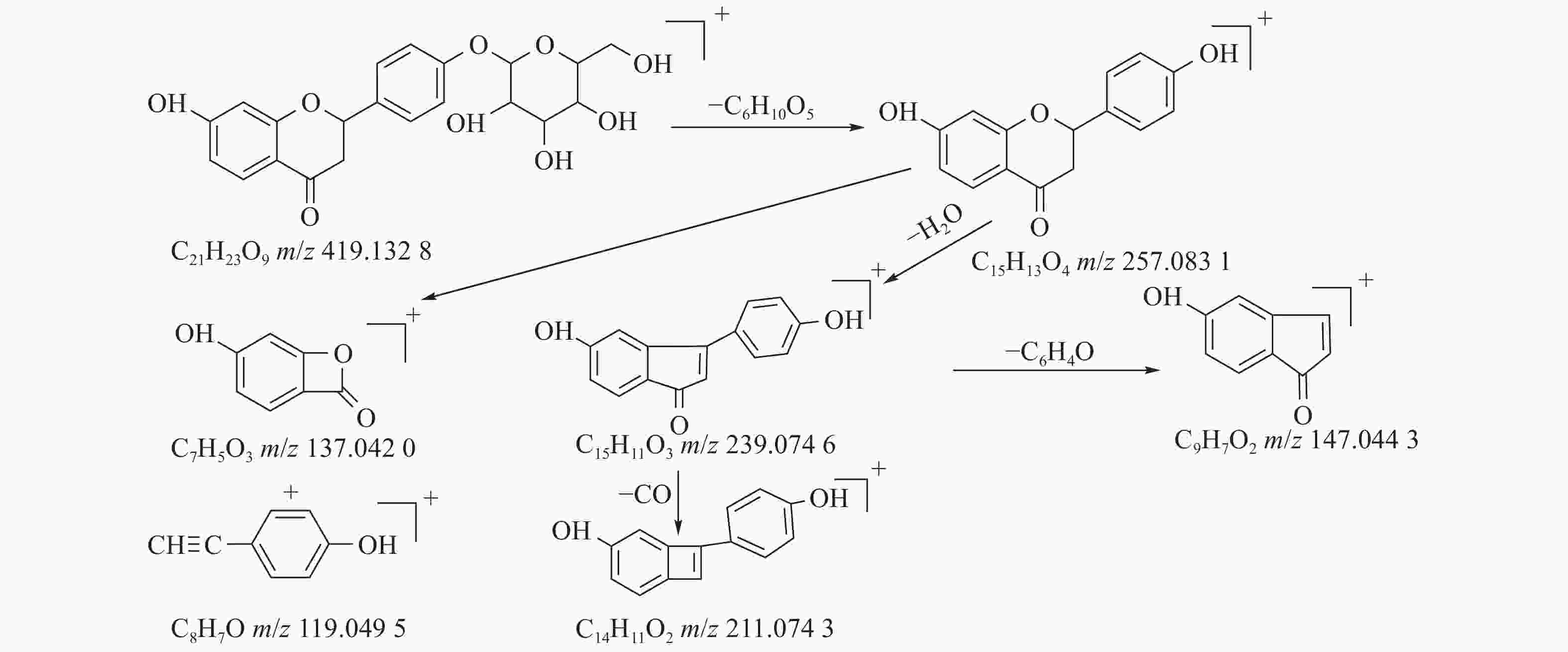
甘草中成分繁多,黄酮类是其主要成分之一,31号峰在正离子模式下裂解途径如下:其母离子峰m/z 419.1328[M+H]+首先脱去一分子的脱水葡萄糖,得到m/z 257.0831[M+H-C6H10O5]+的一个碎片离子峰;其碎片离子进一步失去一个H2O分子,得到碎片离子m/z 239.0746[M+H-C6H10O5-H2O]+;碎片离子再一次分别脱掉一个CO和C6H4O,产生m/z 211.0743[M+H-C6H10O5-H2O-CO]+和m/z 147.0443[M+H-C6H10O5-H2O-C6H4O]+的两种碎片离子峰,在逆狄尔斯-阿尔德反应(RDA)的裂解下,碎片离子峰m/z 257.0831[M+H-C6H10O5]+产生了两种碎片离子,分别是m/z137.0240[M+H-C6H10O5-C8H8O]+和m/z119.0495[M+H-C6H10O5-C7H4O3]+,通过参考甘草苷化合物的碎片离子(m/z),对照对照品二级质谱碎片图,如图4所示,由此可以鉴定出该化合物为甘草苷,裂解途径如图5所示。
-
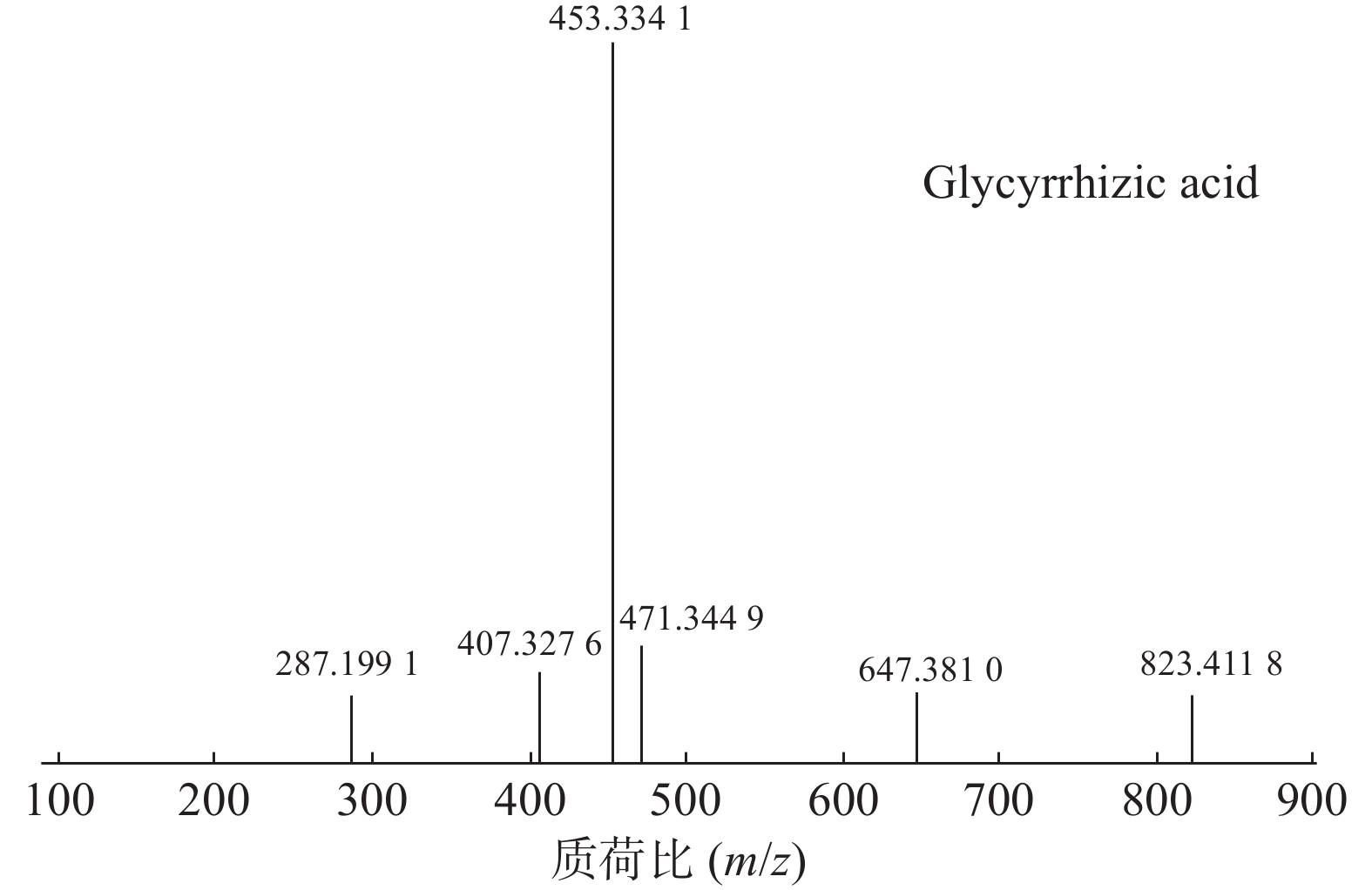
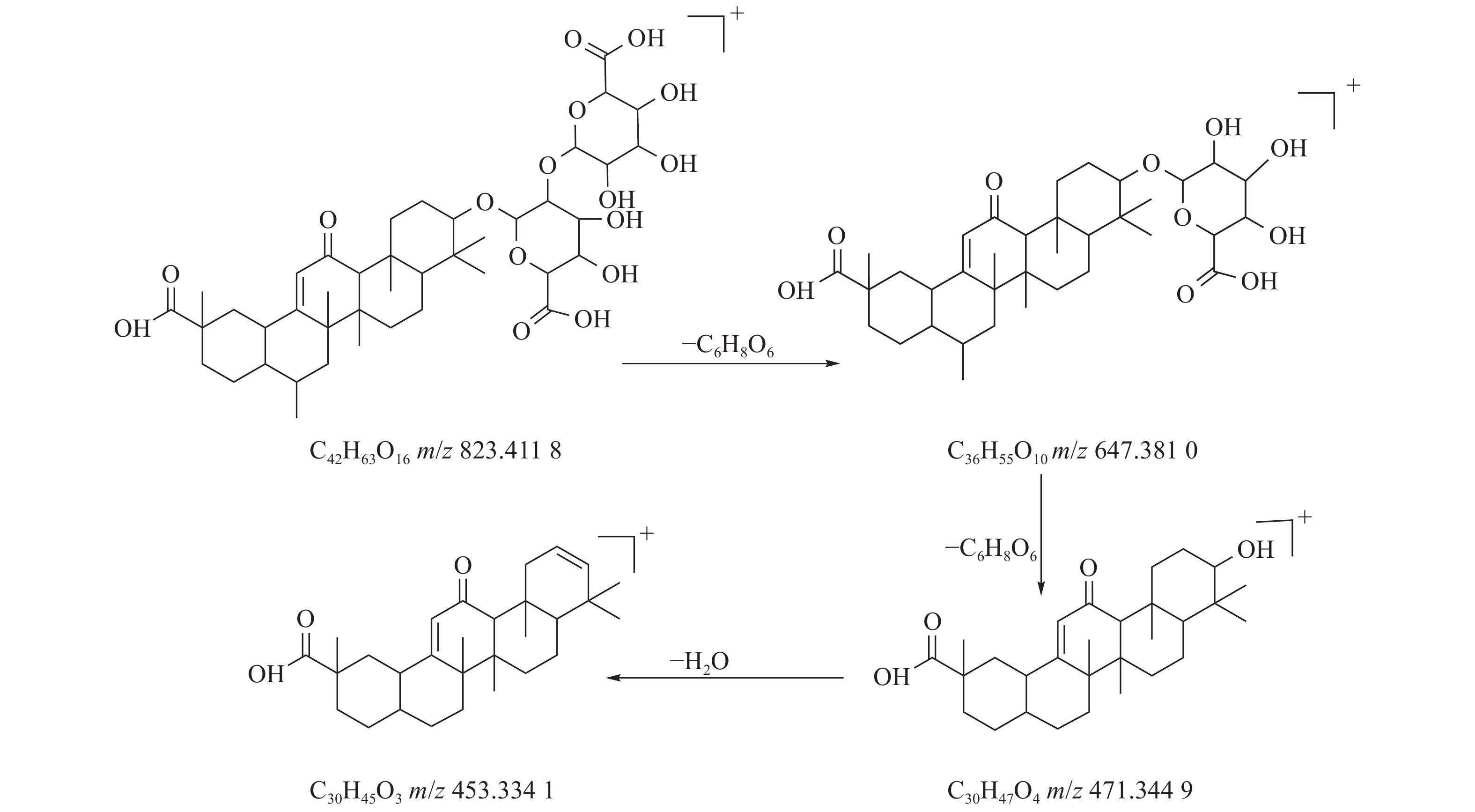
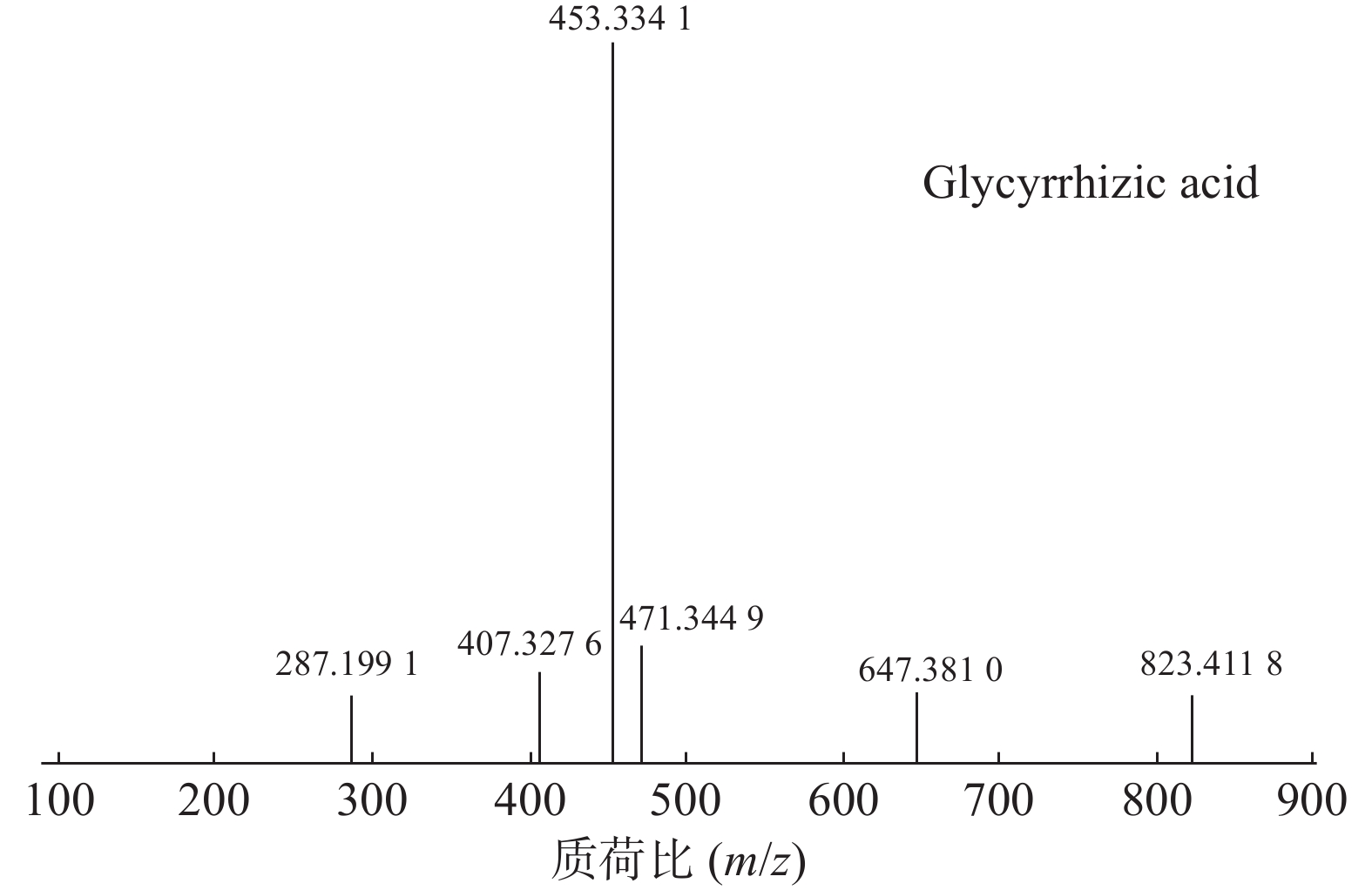
甘草酸属于三萜皂苷类,在甘草、苦杏仁中都有其成分。在正离子的扫描模式下,43号峰的母离子峰为m/z 823.4118[M+H]+,母离子峰首先脱去一分子的糖醛酸,得到碎片离子m/z 647.3810[M+H-C6H8O6]+,然后又脱去一个糖醛酸,得到碎片离子m/z 471.3449[M+H-2C6H8O6]+,接着又失去一分子的H2O,得到m/z 453.3341[M+H-2C6H8O6-H2O]+的碎片离子峰,本研究参考化合物甘草酸的碎片离子(m/z),以及参照对照品二级质谱碎片图,如图6所示,根据该类化合物的相关裂解规律可以判断为甘草酸,裂解途径如图7所示。
-
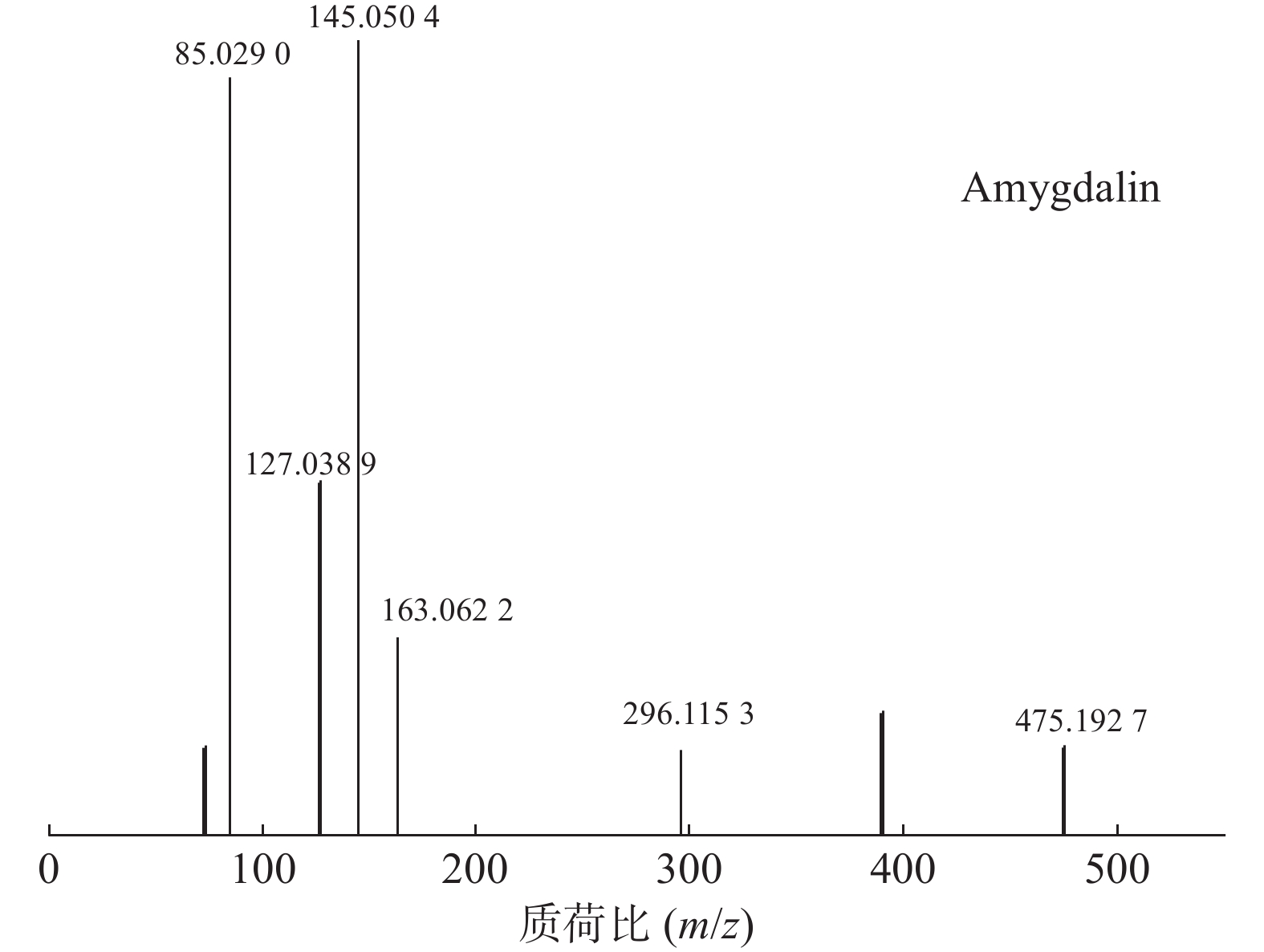
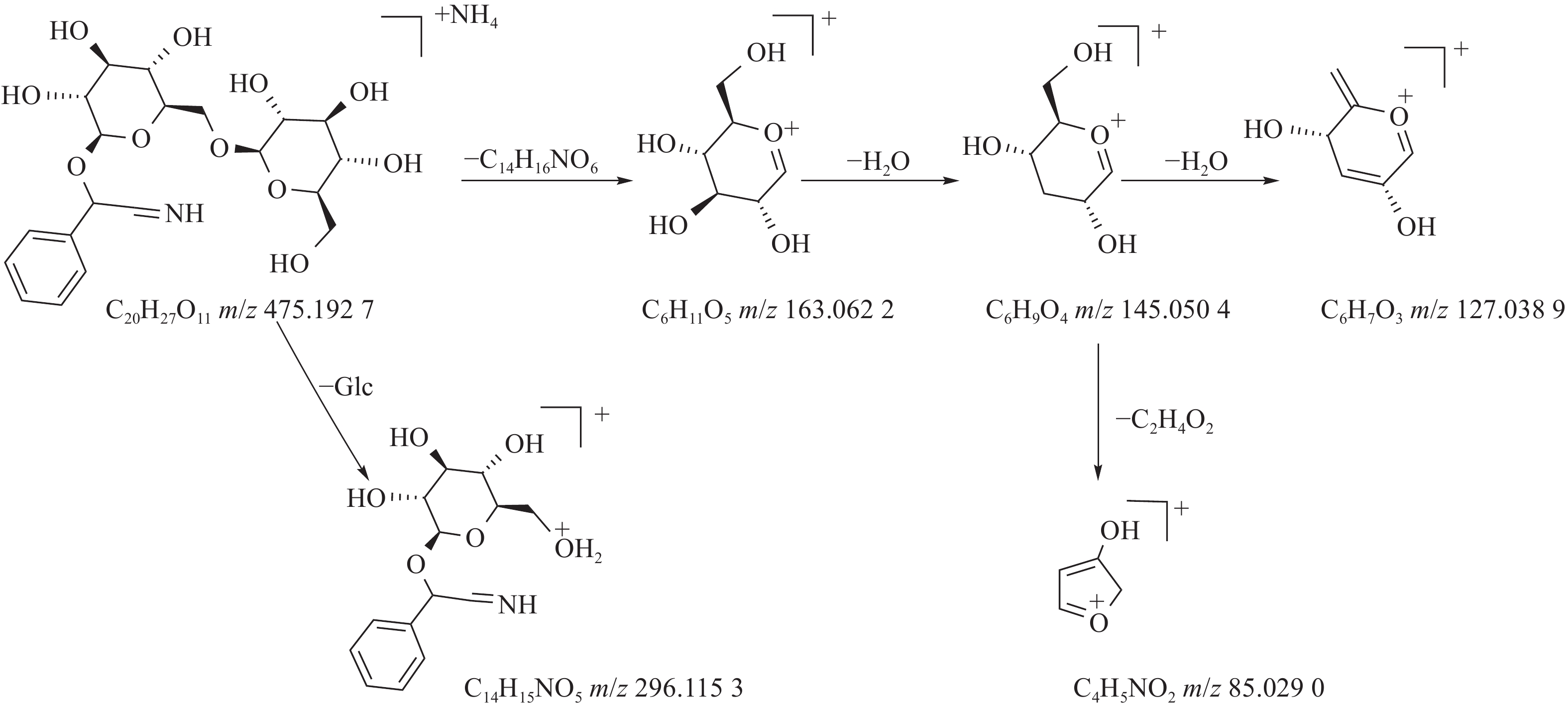
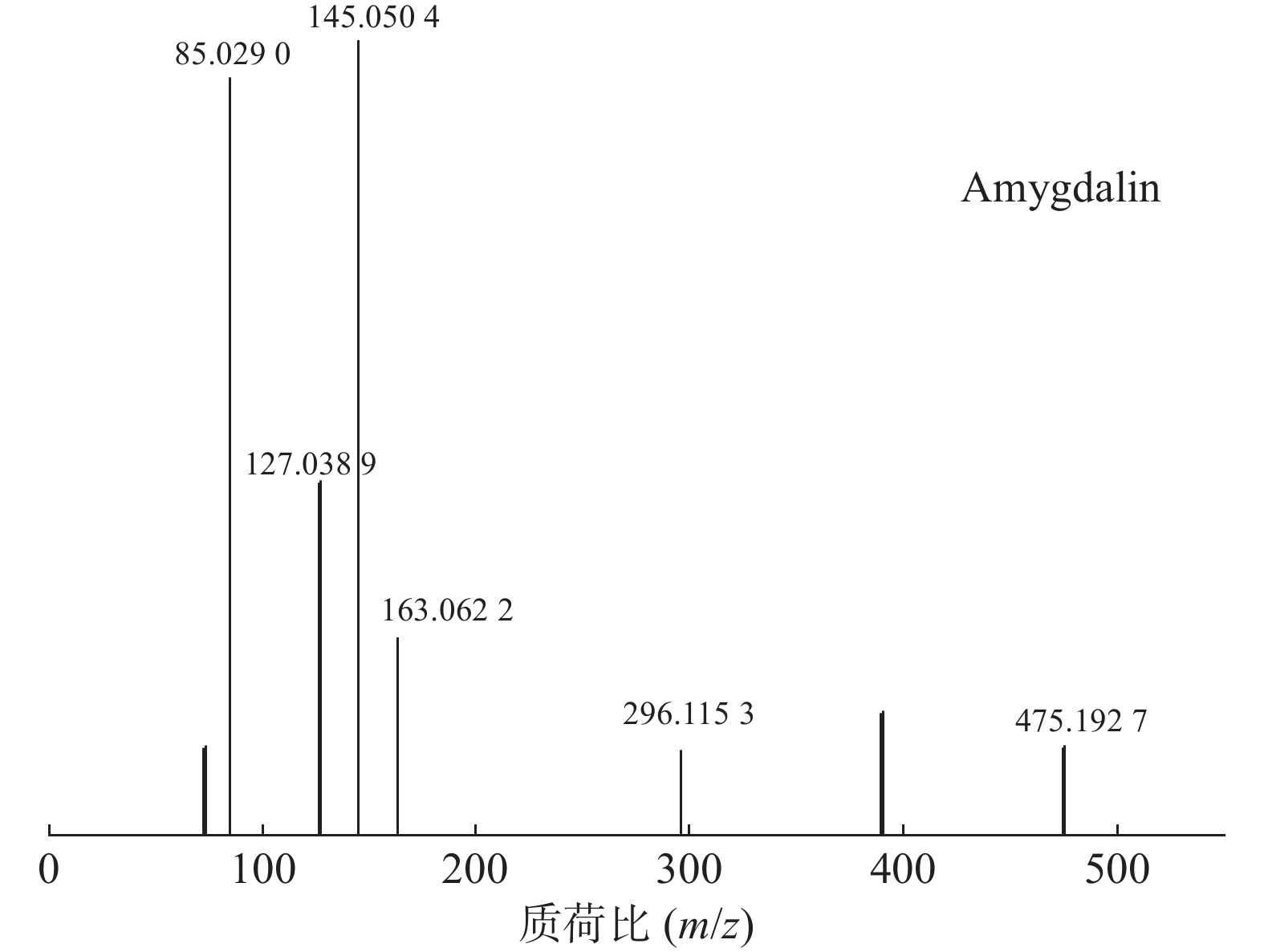
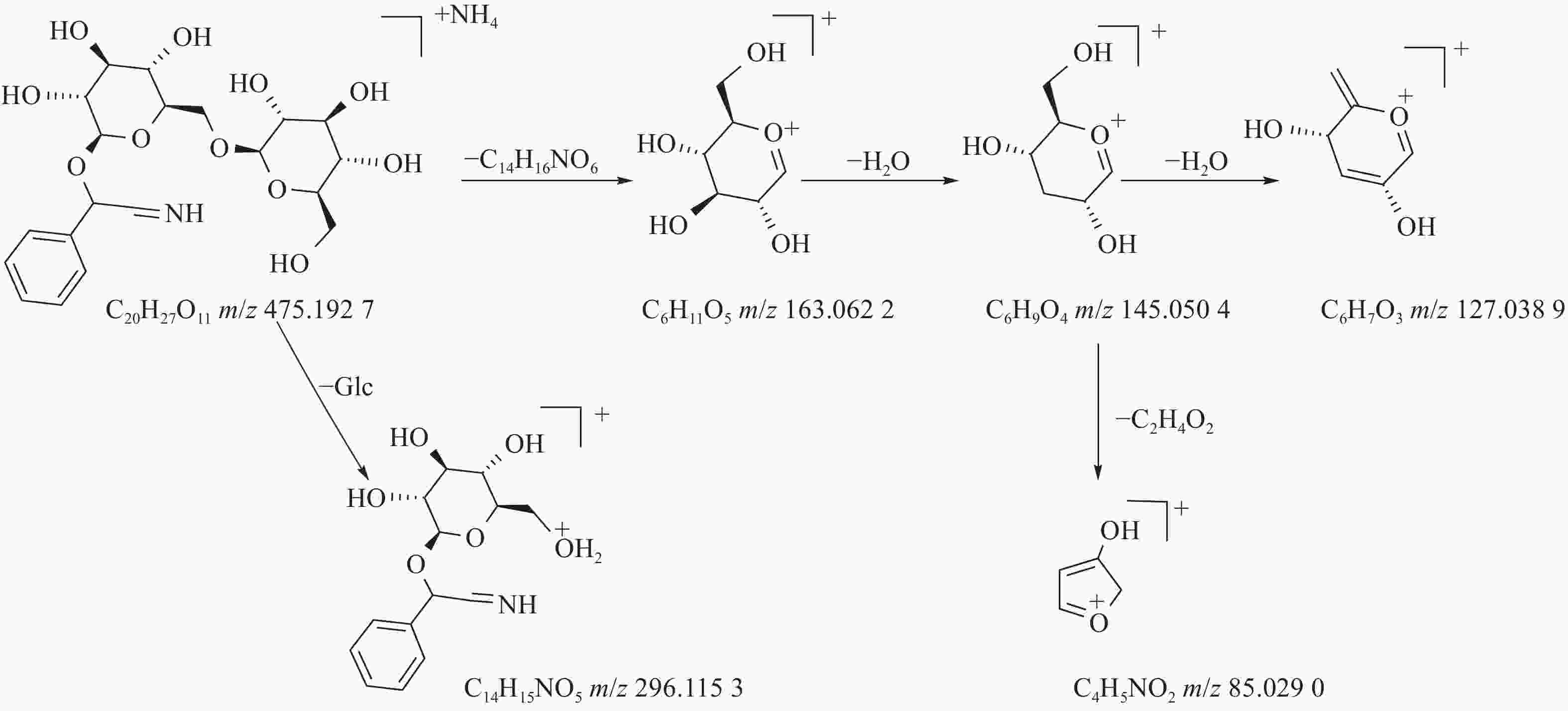
苦杏仁属传统常用中药,苦杏仁苷是苦杏仁中的一种主要成分。在正离子模式下,化合物23的分子式为C20H27NO11,由m/z 458.1649([M+H]+)和m/z 475.1927([M+NH4]+)的两个峰可知,m/z 296.1153处的峰值归因于[M+NH4-Glc]+。将可能的碎片化途径与参考数据进行比较[17-19],并结合ESI(+)模式保留时间与参照对照品二级质谱碎片图(见图8),结果表明化合物23为苦杏仁苷,是苦杏仁中存在的一种化学成分,其他特征碎片以及可能的碎片裂解途径如图9所示。
-
麻杏石甘汤由麻黄、杏仁、石膏和甘草组方而成,方中麻黄主要的生物碱类化合物具有止咳平喘、镇痛等临床疗效,甘草中的黄酮类成分有明显的抗炎、抗菌、抗肿瘤的功效,此外,方中甘草酸和苦杏仁苷等有效成分均有其平喘、止咳的作用。麻杏石甘汤组方简单,药效显著,在新冠肺炎不同病理过程的治疗阶段都有其身影,但是已有研究对该方的化学成分的深入研究尚浅,因此,明确麻杏石甘汤的主要化学成分对于揭示其组方原理,阐明作用机制与药效物质基础具有一定的参考价值。
本研究采用 UPLC-QTOF/MS 技术,在短时间内对麻杏石甘汤的化学成分进行了定性分析,一共鉴定出了59种有效的化学成分,该方法分离速度快,分辨率高且分析时间短。采用二级质谱分析验证了麻黄碱等主要成分的质谱碎片裂解规律,为麻杏石甘汤的入血成分的快速鉴定提供方法学基础。本研究为麻杏石甘汤抗新冠肺炎药效物质基础及其作用机制的深入研究奠定基础。
Analysis of the chemical constituents of Maxing Shigan decoction by UPLC-Q-TOF/MS
doi: 10.12206/j.issn.2097-2024.202306028
- Received Date: 2023-06-14
- Rev Recd Date: 2023-11-06
- Available Online: 2024-02-26
-
Key words:
- Maxing Shigan decoction /
- chemical composition /
- COVID-19 /
- UPLC-Q-TOF-MS
Abstract:
| Citation: | ZHAO Xue, GU Yanqiu, CHU Haowen, WU Caisheng, LI Gao, CHEN Xiaofei. Analysis of the chemical constituents of Maxing Shigan decoction by UPLC-Q-TOF/MS[J]. Journal of Pharmaceutical Practice and Service. doi: 10.12206/j.issn.2097-2024.202306028 |








 DownLoad:
DownLoad: