-
多细胞生物的出现是进化过程的一个里程碑事件,器官大小的精密调控对于多细胞动物的发育和再生过程都非常重要。Hippo 信号通路是多细胞动物体内调节细胞增殖和凋亡的主要信号通路之一,在组织稳态维持和器官大小调控中起着关键作用 [1] 。
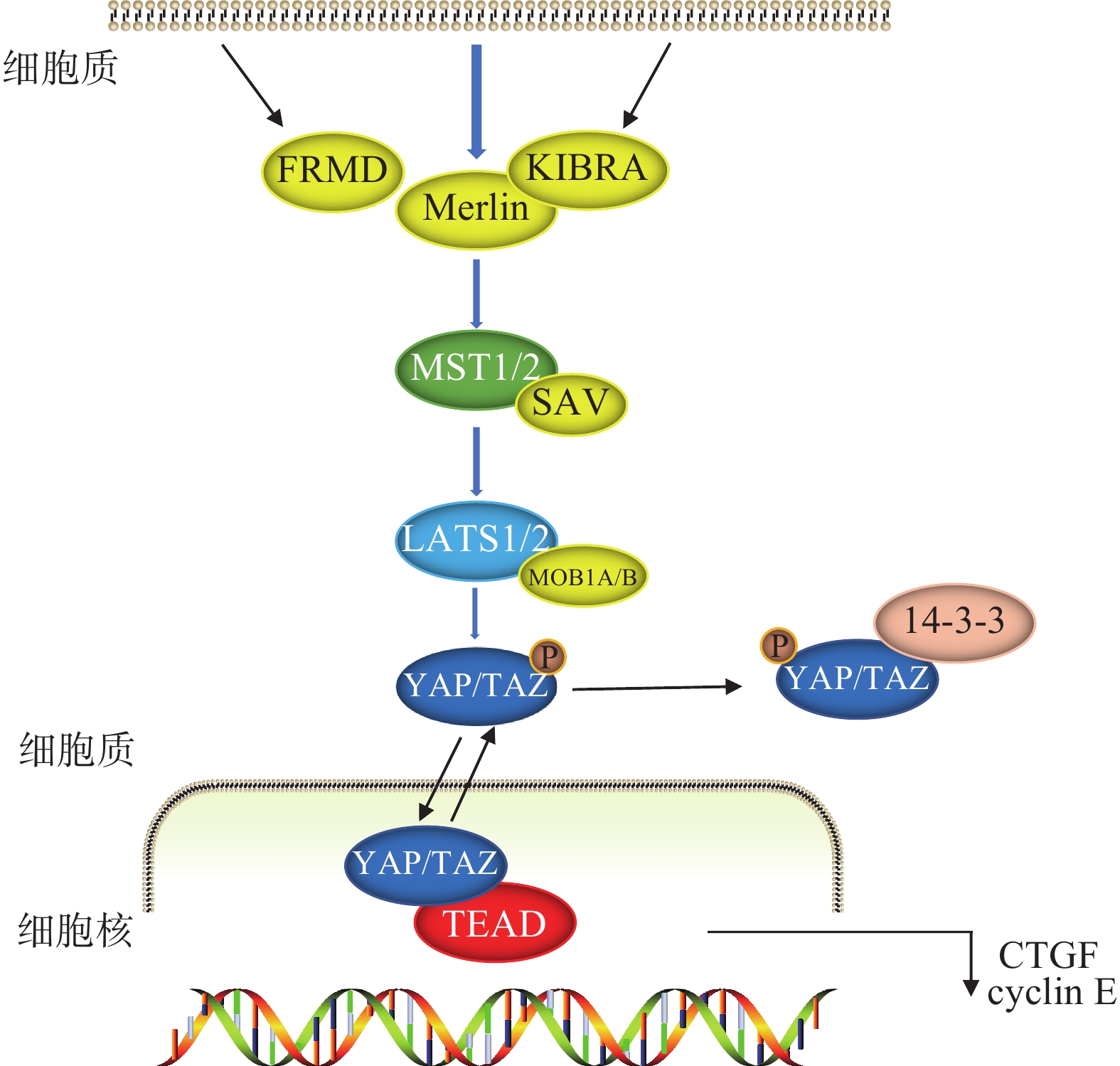
Hippo信号通路由一系列蛋白激酶及转录共激活因子组成,可调节细胞增殖和凋亡[2-3],在胚胎发育和组织器官生长发育中至关重要[4]。以哺乳动物为例,当Hippo信号通路激活的时候,在上游信号输入因子的作用下,MST1/2被磷酸化后与SAV结合,进一步激活LATS1/2以及MOB1A/B,两者结合后将下游的转录辅因子YAP/TAZ磷酸化,随后与14-3-3蛋白结合,最后被降解或隔离在细胞质中,无法进入细胞核进行转录,从而阻止细胞增殖。当Hippo信号通路被阻断或失活时,YAP/TAZ进入细胞核,与TEAD形成功能杂交转录因子,启动促增殖和促生存基因,促进细胞增殖(图1)[5-7]。
作为转录共激活因子,YAP/TAZ不具有DNA结合域,它们通过与转录因子TEAD1-4相互作用促进下游,如CTGF、CYR61、ANKRD1等靶基因的表达,从而启动YAP/TAZ的促增殖和抗凋亡功能,激活细胞周期和细胞存活基因的转录,促进器官的生长发育[8]。
当Hippo信号通路各组分处于相对平衡状态时,机体可保持正常生理功能,严格控制细胞的增殖与凋亡,进而调控器官的形状与大小。最近研究表明, Hippo 信号通路在心脏、肝脏、肌肉等多种组织器官的发育与再生中发挥重要作用。然而大多数哺乳动物的再生潜力有限,只有少数器官具有一定的再生能力。本文简要概述Hippo信号通路在器官再生中的作用机制,阐明其在器官发育、再生重塑中的作用,总结靶向 Hippo信号通路的小分子及多肽抑制剂,为临床治疗器官损伤等疾病提供理论依据。
-
研究表明,Hippo 信号通路通过调节心肌细胞增殖与分化调控心脏大小与功能,维持心脏正常的生长发育 [9-10]。Hippo信号通路的主要效应因子YAP对心脏发育和出生后心肌细胞增殖都很重要。YAP 激活不但可以调控心脏在胚胎期的形态发生与发育[11],促进新生和成年心肌细胞的增殖[12-14] ,而且可以提高新生小鼠心脏的再生能力[13,15]。
在小鼠心脏干细胞中,早期YAP缺失会引起细胞增殖减少,形成异常薄的心肌,导致心肌发育不良和收缩功能不全 [12]。同理,胎儿心肌细胞中YAP缺失会导致心肌细胞增殖下降和心脏发育不全[8]。YAP过表达可以使得成年哺乳动物的心肌细胞重新进入细胞周期,增强心肌细胞增殖,导致心肌异常增厚和小梁层扩张[11]。YAP的激活刺激了新生儿和成人心肌细胞的增殖,延长心脏再生窗口,增强损伤后的心脏修复能力[13]。
Heallen等[9,15]采用基因敲除的方法敲除胚胎小鼠心脏中的 SAV1、MST1/2 和Lats2,发现心肌细胞数量增加、小梁扩张和心肌增厚,心脏异常增大。Xiang等[16]发现转录因子Tbx20 在成年心肌细胞中过表达可降低 YAP 的磷酸化,激活多种心脏增殖途径,促进心肌细胞增殖和心脏修复并改善心功能。Li等[17]发现出生后特异性的敲除小鼠 α-连环蛋白可提高成年心肌细胞增殖能力。
Tian等 [18]证实MicroRNA簇miR302-367可以有效抑制Hippo通路,促进 YAP入核,诱导成年心肌细胞增殖并促进心脏再生。实验表明基于MicroRNA的治疗方法能够通过心肌细胞增殖,促进哺乳动物心脏修复和再生。Gabisonia及其团队[19]用 miRNA-199a 干扰 Hippo通路,证实使心肌再生并改善了猪心脏的收缩功能,但在这项研究中,心肌细胞失控,导致大多数动物在治疗2个月内死亡。
Li等[20]发现抑菌素M (OSM)是新生儿心脏再生过程中的调控因子,糖蛋白130(GP130)作为OSM的共受体,通过Y357磷酸化激活 YAP 来促进心肌细胞的增殖,改善心肌损伤后再生。该研究表明, GP130是改善心脏损伤、促进心肌再生的潜在治疗靶点。Hara等[21]和Ito等[22]发现新型含氟化合物TT-10(C11H10FN3OS2)可以增强YAP-TEAD活性,进而保护心脏免受缺血性损伤、促进心肌细胞增殖、改善小鼠心肌梗死后的心功能。
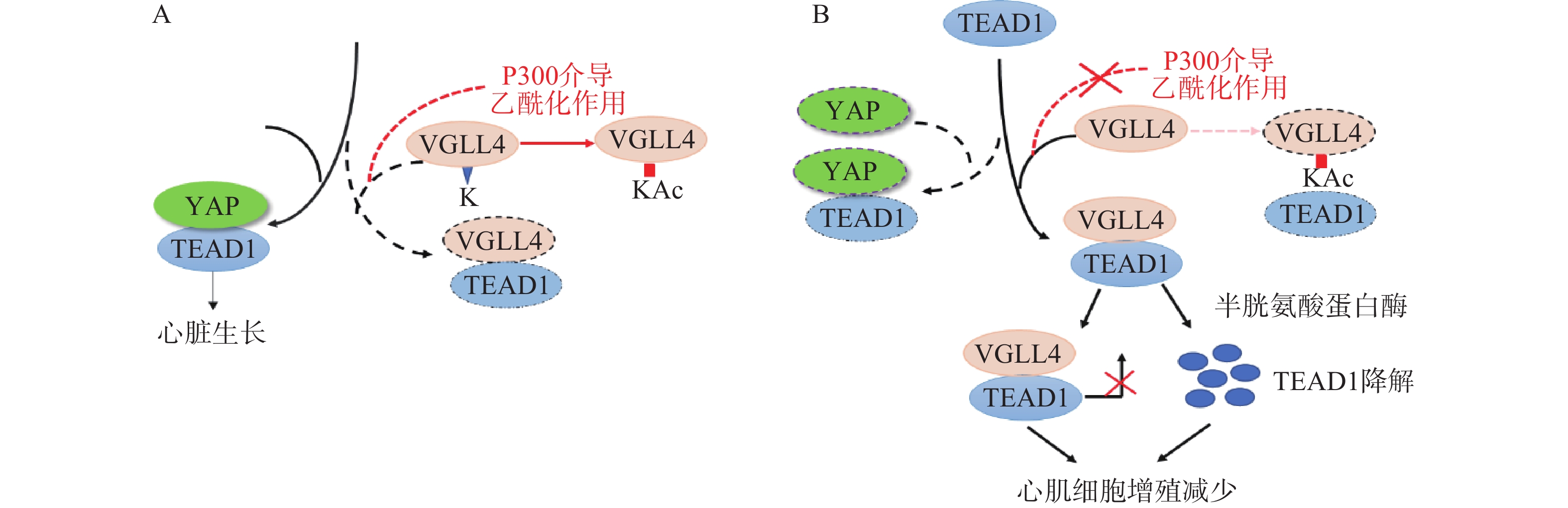
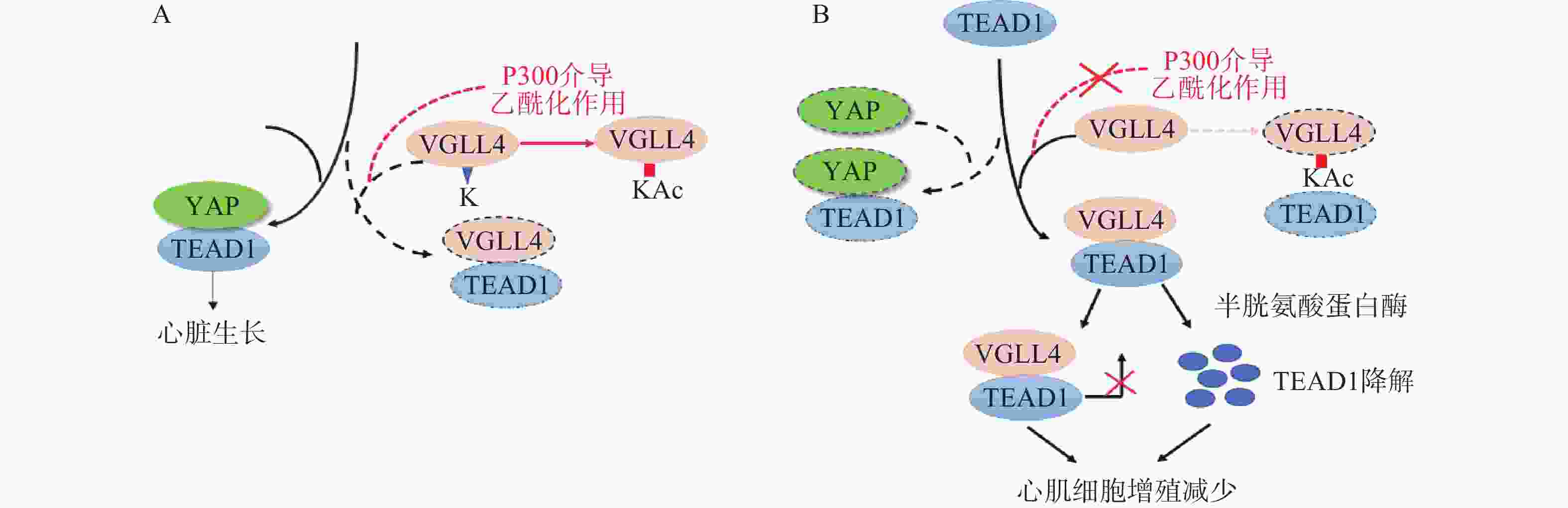
Lin等[23]在研究影响YAP有丝分裂活性的因素时发现,VGLL4的乙酰化可调节VGLL4对TEAD1-YAP的拮抗作用,影响新生儿心脏生长。研究表明,VGLL4的K225位点乙酰化降低了新生儿心脏中VGLL4与TEAD的相互作用, TEAD1-YAP相互作用促进心肌生长。通过K225R突变降低VGLL4乙酰化作用,促进TEAD1-VGLL4相互作用,抑制了TEAD1-YAP相互作用,促进TEAD1降解,从而使心肌细胞增殖减少(图2)。
Hélène Adihou等[24]报道了一种基于VGLL4-TEAD复合物晶体结构设计的高透膜性的蛋白模拟物,该蛋白模拟物4E以转录辅助因子VGLL4的螺旋片段(203–256)为模板进行环化得到。4E与TEAD共晶结构表明其以预期的方式与TEAD结合,从而抑制TEAD-VGLL4相互作用。实验发现Tat标记的拟蛋白(peptide 7)促进人心肌细胞中TEAD靶基因的表达,并促进幼年大鼠心肌细胞中YAP核易位和细胞周期活动,这是心肌细胞增殖所需的重要特征。
-
肝的再生能力极强,在正常情况下99%的肝细胞处于G0期,在急性肝损伤或肝大部分切除后肝再生能力出众。研究表明Hippo 信号通路在肝脏发育、再生、肝细胞生长和肝癌发生发展等过程中都发挥了非常重要的作用。
肝细胞增殖受到Hippo通路活性的调控。在肝再生早期Hippo 通路信号受到抑制, YAP 活性增加;当肝再生终止后,MST1/2、LATS1/2 和非磷酸化 YAP 恢复到正常水平。YAP过表达可导致肝细胞增殖增加、肝细胞过度生长和肝癌的发展,肝体积在几周内增加约4倍;在缺乏Yap/Taz的情况下,肝再生严重受损,肝星状细胞的增殖扩张被阻断[25-26]。
Hippo通路上游调控因子MST1/2 、LATS1/2、Nf2和SAV1的急性缺失会导致YAP水平升高,肝细胞增殖,肝质量增加,进而引起肝肿大并可能导致肝癌[27-29]。LATS1/2敲除会导致未成熟的胆管上皮细胞的急速扩增、肝肿大并最终导致小鼠死亡。MST1/2活性调节YAP水平, MST1/2急性失活后YAP蛋白水平升高,敲除MST1/2对对乙酰氨基酚诱导的肝损伤有明显的保护作用 [30]。
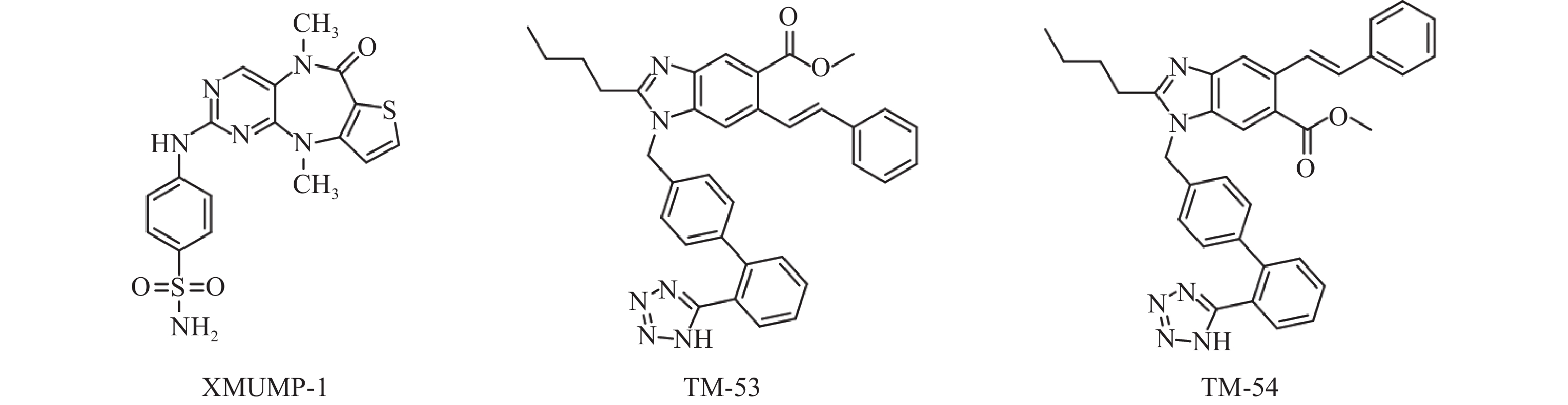
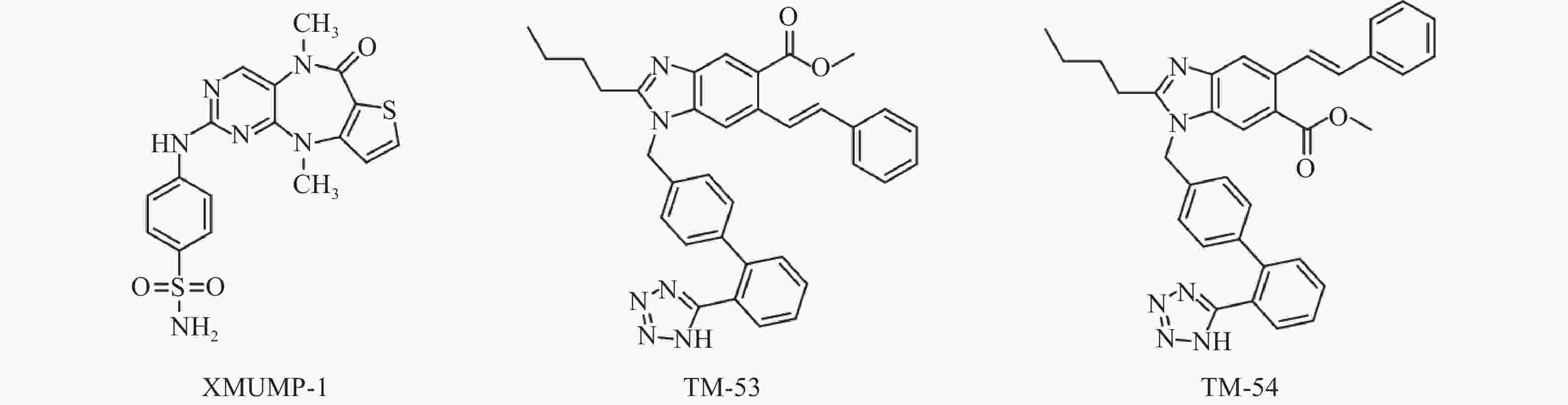
Fan等[31]发现使用MST1/2抑制剂XMUMP-1(图3),可激活下游效应YAP相关蛋白,促进细胞生长;也可以防止MST2过表达引起的细胞死亡,并导致核YAP募集增加,显著改善PHx(70%肝脏部分切除术)后小鼠的肝再生。因此利用XMUMP-1对Hippo信号通路进行调控,可能为肝再生研究和组织修复提供新的治疗选择。
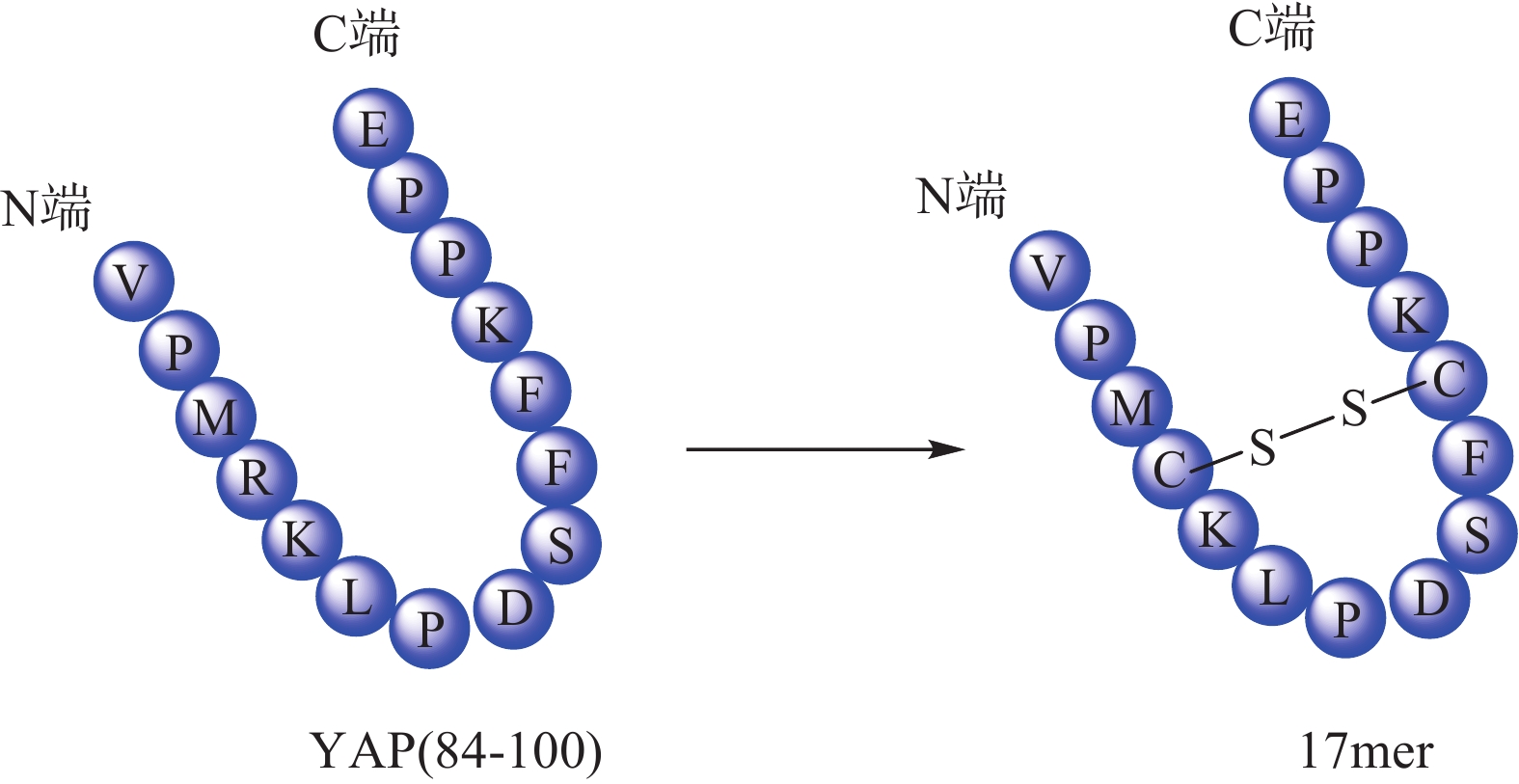
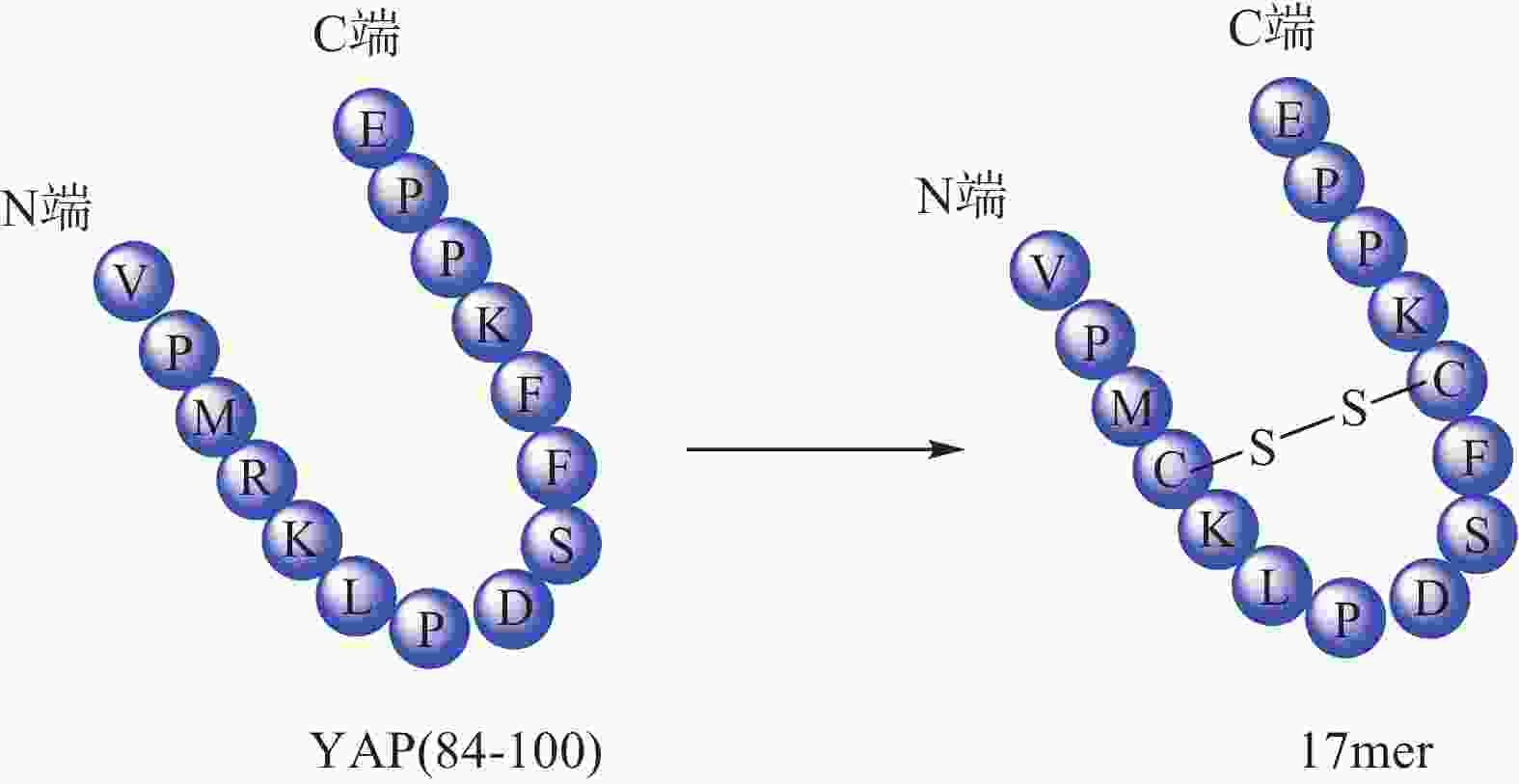
Zhou等[32]根据YAP-TEAD相互作用特点,以YAP为模板肽设计了一种环肽(17mer) (图4),直接靶向TEAD来阻断YAP-TEAD复合物的形成,提示开发YAP-TEAD相互作用抑制剂是一种有前途的抗增殖策略。Liu等[33] 通过筛选发现卟啉类化合物维替泊芬(verteporfin, VP)可以影响YAP的表达,VP能够与YAP选择性结合并改变其构象,抑制YAP-TEAD相互作用,从而抑制下游靶基因转录。VP不仅对 YAP过表达或内源性YAP激活导致的肝过度生长有抑制作用,还对NF2缺失造成的小鼠肝癌也有一定治疗作用。
-
肌肉再生是依赖于干细胞并涉及几个步骤的复杂过程,肌肉组织再生主要通过肌肉干细胞的自我更新来实现[34]。研究发现,Hippo信号通路可以促进骨骼肌肉卫星细胞和成肌细胞增殖,调控成肌细胞分化,并促进骨骼肌蛋白合成。
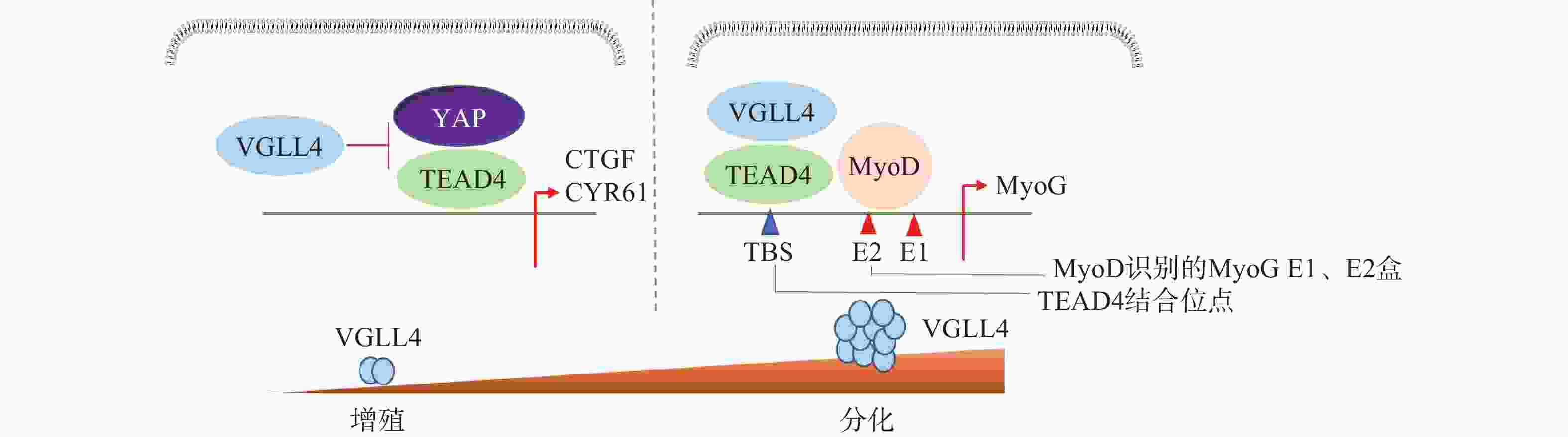
骨骼肌的形成受到肌形成调节因子的精密调控。成肌细胞决定蛋白D (MyoD)是重要的生肌决定因子,成肌细胞决定蛋白G(MyoG)控制成肌细胞分化形成肌纤维。在YAP敲除的小鼠中,YAP的靶基因CTGF、ANKRD1均下调,肌肉蛋白合成速率下降,肌肉比重明显下降,小鼠的肌纤维变细[35]。YAP/TAZ表达增加可以使骨骼肌蛋白合成增加,促进肌肉卫星细胞和成肌细胞增殖;过表达YAP会明显地抑制成肌细胞的分化,而肌纤维面积变大,小鼠体重增加[36]。在成肌细胞中TAZ通过与TEAD4相互作用发挥作用,单独敲除TEAD4可显著降低MyoD的表达,导致肌管变短变薄[37]。
Park等[35]发现使用新型TAZ调节剂 TM-53和TM-54(图3),可增加TAZ核定位,促进MyoD诱导的肌源性基因表达,从而增强成肌细胞的分化。此外,TM-53和TM-54还能减轻损伤后小鼠体外肌纤维损伤,显著增强肌肉再生,改善肌肉活性并提高小鼠的运动能力,提示TM-53和TM-54在治疗肌肉功能障碍方面具有一定作用。
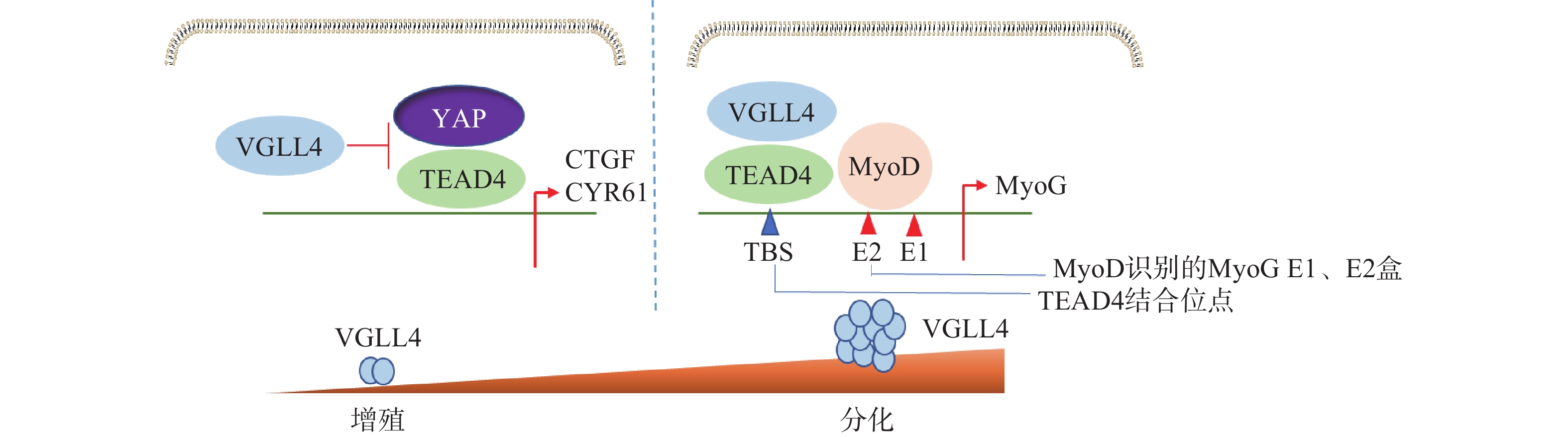
Feng等[38]研究表明,VGLL4在不同阶段以YAP依赖或者不依赖的方式调控肌肉再生。肌肉干细胞中特异性地敲除VGLL4能够显著促进其增殖,抑制分化,导致肌肉卫星细胞增殖增加,成肌细胞分化受损,最终使得肌肉再生过程延迟。在肌肉再生早期,失活VGLL4可以使YAP-TEAD4复合物稳定存在,促进成肌细胞增殖。在肌肉再生后期,VGLL4作为MyoD的共激活剂,通过稳定MyoD-TEAD4的相互作用,在促进肌肉干细胞分化启动过程中发挥重要作用(图5)。研究发现VGLL4是一种调控分化阶段肌肉再生的新型激动剂,这为研究VGLL4对肌肉再生作用提供了新的思路并打开新的治疗前景。
-
皮肤是保护机体免受外部损伤的最大器官,通过基底膜分为表皮层及真皮层。表皮通过不断更新来维持皮肤的动态平衡,表皮组织的自我更新和伤口愈合主要依赖于表皮干细胞[39]。
YAP主要分布于皮肤基底细胞的细胞核中,是表皮干细胞增殖和组织扩张的关键调节剂,对表皮稳态的维持和伤口愈合都特别重要[40-41]。在小鼠皮肤表皮干细胞中,敲除YAP会导致严重的表皮发育不全,小鼠的表皮变薄,角质层减少,导致表皮组织形成不足,无法维持表皮形态[42]。在成年小鼠皮肤中缺失YAP和TAZ可轻微影响基底层细胞的增殖,并引起毛发脱落[41]。相反,Sav1突变体中YAP的过度激活导致基底干细胞过度增殖,从而导致皮肤增厚,皮肤过度增生并伴有不成熟分化,最终导致鳞状细胞癌[43]。
-
综上所述,Hippo 信号通路与人类组织器官大小调控与再生密切相关。近年来,关于Hippo 信号通路在器官再生过程中的作用机制研究取得了一些进展,同时也确认了一些影响Hippo信号通路的相关靶点,但是人们对 Hippo 信号通路的认识还不够完善,比如需进一步探究如何精细调节 Hippo 通路信号及影响因子等。另外,Hippo 信号通路与其他信号通路在调控器官再生相关机制中既有交叉又有重合,将来的研究需要考虑这些信号通路的参与所带来的干扰。
近期研究发现了很多潜在化合物能够调节Hippo信号通路的活性,但是大部分药物的研究局限于动物试验阶段,进入临床试验的药物比较少。同时也产生了很多新的问题,很多药物的作用靶点缺乏特异性,可能会带来其他不良反应,因此有必要进行进一步的研究。
Research progress on the mechanism of Hippo signaling pathway in organ regeneration
doi: 10.12206/j.issn.2097-2024.202301008
- Received Date: 2023-01-10
- Rev Recd Date: 2023-06-07
- Publish Date: 2023-08-25
-
Key words:
- Hippo signaling pathway /
- organ regeneration /
- target /
- inhibitor
Abstract: Hippo signal pathway is one of the main signal pathways regulating cell proliferation and apoptosis in multicellular animals, which plays a vital role in the maintenance of tissue homeostasis and the regulation of organ growth. Most mammals have limited regenerative potential, and recent studies have shown that Hippo signal pathway is critical in the regeneration of various tissues and organs. The role of Hippo signaling pathway in organ regeneration and the research progress of related targets were introduced, the mechanism of Hippo signaling pathway promoting regeneration analyzes were aralyzed in this review, which provide theoretical reference for the treatment of diseases related to organ regeneration.
| Citation: | CHEN Shuai, LI Anpeng, LI Xingxing, ZHAO Qingjie, ZOU Yan. Research progress on the mechanism of Hippo signaling pathway in organ regeneration[J]. Journal of Pharmaceutical Practice and Service, 2023, 41(8): 472-477. doi: 10.12206/j.issn.2097-2024.202301008 |







 DownLoad:
DownLoad: