-
急性冠脉综合征(ACS)病理基础是冠状动脉粥样硬化斑块破裂或侵袭,继而形成不同程度的阻塞性血栓,抗血小板治疗是其治疗的基础,可降低患者血栓事件的发生率[1]。目前我国临床上普遍使用的口服抗血小板药物包括氯吡格雷和替格瑞洛,这类药物通过抑制血小板表面的ADP P2Y12受体发挥作用。研究表明与氯吡格雷相比,替格瑞洛可降低心血管死亡的发生率且不会增加主要致命性出血的风险[2]。近年来国内外相关指南将替格瑞洛推荐作为急性冠状动脉综合征抗血小板治疗的一线用药,尤其对非ST段抬高型ACS患者建议首选替格瑞洛联合阿司匹林进行抗血小板治疗[3-7]。
替格瑞洛虽不受CYP2C19基因多态性影响,但文献报道与替格瑞洛转运受体、作用靶点以及血小板膜受体相关的基因多态性(如SLCO1B1、UGT2B7、P2Y12、PEAR1、ITGA2B、ITGB3等)可能会影响其某些药动学参数以及药效[8-13]。相关基因多态性是否会影响替格瑞洛在中国ACS患者体内的抗血小板效果尚不清楚。本研究收集ACS患者病例,通过全外显子测序法探讨与替格瑞洛有关的转运蛋白、作用靶点以及血小板膜表面受体相关编码基因的多态性,分析基因多态性与替格瑞洛体内抗血小板聚集效果的相关性,为替格瑞洛的临床个体化用药提供参考。
-
选取2018年3月至12月于福建省某三甲医院给予替格瑞洛常规治疗的ACS患者。纳入标准:①诊断为ACS的患者;②年龄≥18周岁,男女不限;③PCI术后阿司匹林100 mg 一天一次、替格瑞洛90 mg一天两次双联抗血小板治疗至少维持1年;④入院后常规给予替格瑞洛抗血小板治疗至少7 d;⑤同意签署知情同意书。排除标准:①免疫缺陷性疾病病史,包括HIV检查结果阳性;②乙肝表面抗原(HBsAg)或丙肝检查结果阳性;③长期使用CYP3A诱导剂、治疗窗狭窄的CYP3A底物、CYP3A抑制剂;④肝功能异常和肾功能异常;⑤收缩压>180 mmHg或舒张压>110 mmHg;⑥存在以下情况:如一个月内进行过大手术,患有中重度肝病,活动性出血,既往颅内出血史,过去6个月内的消化道出血,超敏反应等。
用药方案:入院后常规接受阿司匹林100 mg一天一次、替格瑞洛90 mg一天两次双联抗血小板治疗。
-
采集:患者口服替格瑞洛至少7 d后收集清晨空腹静脉血样2.7 ml,测定给药后的ADP血小板聚集率;与上述同一时间采集静脉血样4 ml测定基因。
药物基因组学(PG)血样处理及保存:血样采集后,若无法立即处理,可室温放置8 h,或先置于4 ℃冰箱保存24 h;血样离心条件:7500 r/min,4 ℃,10 min。下层血细胞用滴管移至冻存管中,平均分装成2份(set 1和set 2),贴PG标签。冻存管需标注研究对象信息,统一标注为药物-患者PG-set1/2编号-时间点;冻存管保存于−70 ℃冰箱,待基因检测。
药物效应动力学(PD)血样处理及保存:血样采集后需在10 min~4 h内进行血小板聚集率检测。
-
提前制备好富血小板血浆(PRP)、贫血小板血浆(PPP)及ADP诱导剂,放置于−20 ℃冰箱内,实验前5 min复溶备用,每支仅使用1次。通过LBY-NJ4血小板聚集仪(北京泰利康信科技有限公司)检测血小板聚集率。
-
依照海普洛斯实验标准处理流程,对收集的血样进行质检、核酸提取、文库制备和上机测序。再依照海普洛斯数据分析标准处理流程,对原始数据进行质控,参考序列比对,去重复,检测以及对变异结果进行下游分析。最后,利用卡方检验原理验证哈迪-温伯格平衡 (HWE)。P>0.05认为符合HWE。应用在线软件SHEsis (http://analysis.bio-x.cn/myAnalysis.php) 进行连锁不平衡(LD)分析,D'>0.8且r2>0.8提示两位点间存在明确连锁不平衡。
-
①一般资料:性别、年龄、身高、体质量、BMI、吸烟史、饮酒史等;②患病类型:ST段抬高型急性冠脉综合征(STE-ACS)、非ST段抬高型急性冠脉综合征(NSTE-ACS);③合并疾病:高血压、糖尿病、高脂血症等;④生化指标:血红蛋白含量、血小板计数、凝血四项、血脂等;⑤联合用药:他汀类(Statins)、β-受体拮抗剂(β-blocker)、血管紧张素转化酶抑制剂(ACEI)/血管紧张素Ⅱ受体拮抗剂(ARB)、质子泵抑制剂(PPI)等。
-
数据统计分析使用SPSS 24.0软件;计量资料数据用均数±标准差(
$ \bar x \pm s $ )表示,计数资料数据用百分比表示;两组间参数比较采用独立样本t检验,多组间参数的比较采用单因素方差分析,若P<0.05,认为存在显著性差异;将单因素分析中P<0.1的变量进行多因素分析;采用调整混杂因素的线性回归法预测影响替格瑞洛体内抗血小板效果的独立遗传变量。 -
本研究共纳入符合要求的受试者75例,63例男性,12例女性,平均年龄为(60.37±11.70)岁,平均体质量指数(BMI)值为(24.48±3.66)kg/m2。表1列出了75例患者的患病类型、合并疾病以及合并用药情况。
临床因素 均数($ \bar x \pm s $) 例数(n,%) 临床因素 均数($ \bar x \pm s $) 例数(n,%) 患病类型 凝血酶原时间(sec) 11.20±1.46 NSTE-ACS 46(61.3) 凝血酶时间(sec) 18.07±1.67 STE-ACS 29(38.7) 活化部分凝血活酶时间(sec) 28.20±4.07 合并疾病 国际标准化比值(INR) 0.99±.060 糖尿病 24(32.0) 糖化血红蛋白(%) 6.49±1.54 高血压 41(54.7) 合并用药 高脂血症 40(53.3) 质子泵抑制剂 75(100) 吸烟 43(57.3) 他汀类 75(100) 实验室指标 β-受体阻滞剂 50(66.7) 总胆固醇(mmol/L) 4.35±1.12 血管紧张素转化酶抑制剂 55(73.3) 甘油三酯(mmol/L) 1.86±1.44 钙离子通道阻滞剂 15(20.0) 高密度脂蛋白(mmol/L) 1.08±0.27 心功能检测 低密度脂蛋白(mmol/L) 2.87±0.88 左心室射血分数(%) 59.35±7.04 血小板计数(×109/L) 229.95±64.31 血小板功能检测 血红蛋白含量(g/L) 134.55±12.36 MAXADP (%) 9.28±6.32 -
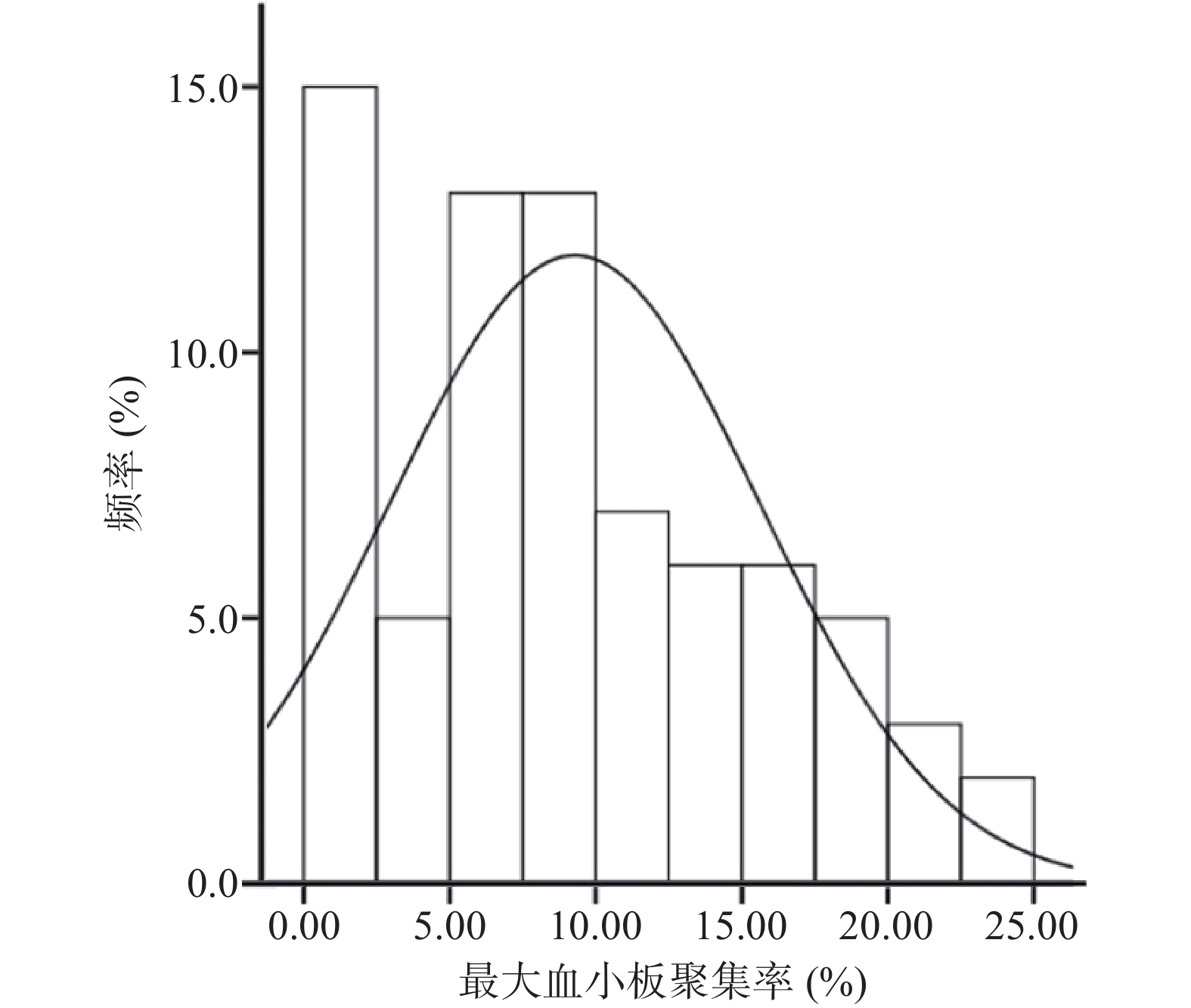
采用MAXADP(即ADP诱导的最大血小板聚集率)反映替格瑞洛的抗血小板效果。当使用的诱导剂为5 μmol/L的ADP时,光学比浊法(LTA)检测到的最大血小板聚集率的治疗范围为MAXADP<50%,当MAXADP>50%时提示血栓形成的风险升高[14]。
本研究入组患者最大血小板聚集率的分布情况如图1所示,结果显示所有患者服用替格瑞洛后的最大血小板聚集率均在治疗范围之内。经K-S检验(Kolmogorov–Smirnov test),最大血小板聚集率基本符合正态分布(P>0.05)。
-
根据文献报道确定与替格瑞洛有关的转运蛋白、作用靶点以及血小板膜受体的编码基因[8-13](基因为SLCO1B1、UGT2B7、P2RY12、PEAR1、ITGA2B、ITGB3),在入组患者全外显子测序的结果中筛选出以上相关编码基因上所有的外显子突变位点以及调控区域突变位点。本研究共筛选出18个突变位点,全部突变均发生在外显子区域。
-
将患者各临床因素与最大血小板聚集率进行单因素分析(见表2)。结果表明,与最大血小板聚集率显著相关的是患病类型,STE-ACS患者的最大血小板聚集率较NSTE-ACS患者高(11.73%±5.55% vs 7.74%±6.35%,P=0.007)。这表明STE-ACS患者服用替格瑞洛后抗血小板聚集效果较NSTE-ACS患者差。
临床因素 组别 例数
(n)MAXADP
(%,$ \bar x \pm s $)P 性别 男 63 9.01±6.20 0.399 女 12 10.70±7.02 年龄(岁) ≥65岁 30 8.33±6.01 0.294 <65岁 45 9.91±6.51 体质量指数(kg/m2) ≥24 43 9.04±6.15 0.706 <24 32 9.60±6.63 高血压 是 41 10.40±6.08 0.092 否 34 7.93±6.44 糖尿病 是 24 9.20±4.48 0.933 否 51 9.32±7.06 高脂血症 是 40 9.38±6.77 0.888 否 35 9.17±5.88 吸烟 是 43 8.79±6.26 0.437 否 32 9.94±6.44 钙离子通道阻滞剂 是 15 7.07±5.88 0.131 否 60 9.83±6.35 血管紧张素转化酶抑制剂 是 55 9.64±6.42 0.422 否 20 8.30±6.10 β-受体阻滞剂 是 50 8.61±6.52 0.195 否 25 10.63±5.80 患病类型 NSTE-ACS 46 7.74±6.35 0.007* STE-ACS 29 11.73±5.54 注:*P <0.05,表示临床因素与患者用药后的最大血小板聚集率有显著相关性 其余临床因素与患者用药后的最大血小板聚集率无显著相关性(P>0.05)。为找出更多可能的影响因素,将显著性水平调整为P<0.1,数据显示有高血压病史的ACS患者平均最大血小板聚集率高于无高血压病史者(10.40%±6.08% vs 7.93%±6.44%,P=0.092),这表明合并高血压有可能会对ACS患者服用替格瑞洛后最大血小板聚集率产生一定的影响。
将血小板计数、低密度脂蛋白水平、高密度脂蛋白水平、糖化血红蛋白含量这四个临床因素作为连续变量,与LTA测得的最大血小板聚集率进行双变量单因素相关分析,得到的结果见表3。由表3可以看出,以上4个临床因素与LTA检测的最大血小板聚集率均不存在显著相关性(P>0.05)。
临床因素 r P 血小板计数 0.190 0.102 总胆固醇 0.066 0.577 高密度脂蛋白 0.58 0.621 低密度脂蛋白 −0.76 0.517 糖化血红蛋白(%) 0.083 0.482 -
将入组的75例患者按照相关突变位点的不同基因型进行分组分析,比较各SNP相应基因型之间平均最大血小板聚集率的差异(见表4)。结果显示18个候选SNPs中SLCO1B1 rs2306283会影响替格瑞洛抗血小板聚集的效果,至少携带一个突变基因G的患者(AG型+GG型)平均最大血小板聚集率显著低于携带野生纯合子AA型的患者(8.07%±6.17% vs 13.88%±6.39%,P=0.042)。其余候选SNPs各基因型与替格瑞洛抗血小板聚集效果均无统计学相关性。这表明SLCO1B1 rs2306283 G 等位基因可能会影响替格瑞洛在体内的转运,进而增强替格瑞洛在体内的抗血小板聚集效果,具体机制有待进一步探究。
基因 基因型 例数 MAXADP
(%,$ \bar x \pm s $)P All W比H/V SLCO1B1
rs2306283AA 7 13.88±6.39 0.124 0.042* AG 32 8.58±6.01 GG 36 9.01±6.38 SLCO1B1
rs4149056TT 65 9.60±6.37 − 0.263 TC 10 7.18±5.98 SLCO1B1
rs2291075CC 29 9.50±5.99 0.903 0.810 CT 29 9.42±6.32 TT 17 8.67±7.12 注:All指的是野生纯合型、突变杂合型以及突变纯合型三组基因型间最大血小板聚集率的比较;W比H/V指的是野生纯合型与突变型(突变杂合型+突变纯合型)两组基因型间最大血小板聚集率的比较 -
将单因素相关性分析中得到的P<0.1的因素即高血压、患病类型以及SLCO1B1 rs2306283 G等位基因纳入多因素线性回归模型中,对高血压、患病类型进行调整,得到SLCO1B1 rs2306283 G等位基因对替格瑞洛抗血小板聚集效果的影响程度。具体结果见表5。
变量 非标准化系数β 标准化系数β t P 高血压 1.791 0.142 1.262 0.221 STE-ACS 3.026 0.235 1.919 0.059 rs2306283(AG+GG) −2.697 −0.125 −1.031 0.306 从表5可知STE-ACS、高血压以及SLCO1B1 rs2306283 G等位基因对最大血小板聚集率影响依次减弱(|β|:0.235>0.142>0.125),但是三者均不会显著影响患者服用替格瑞洛后的最大血小板聚集率(P>0.05),因此,调整高血压、患病类型这两个混杂因素后,SLCO1B1 rs230628 G等位基因并不是影响替格瑞洛抗血小板聚集效果的独立变量。
-
全外显子测序结果显示在SLOC1B1基因外显子区域检测到3个可信度较高的突变位点,分别是rs2306283、rs4149056和rs2291075。
一项体外研究表明rs2306283 G等位基因会降低OATP1B1对利福平的转运活性[15-16]。WEN等[17]研究表明SLOC1B1 388A>G(rs2306283)单核苷酸多态性会引起健康中国志愿者匹伐他汀药动学的显著改变:AG+GG组的Cmax、AUC0–48、AUC0–∞显著高于AA组。目前没有文献报道rs2306283单核苷酸多态性是否会影响替格瑞洛的药动学,但本研究数据表明rs2306283单核苷酸多态性会影响ACS患者服用替格瑞洛后抗血小板聚集效果:AG+GG组的血小板聚集率显著低于AA组,即AG+GG组患者服用替格瑞洛后对血小板的抑制作用较强。因此,我们推测SLCO1B1 rs2306283 G等位基因虽不是影响抗血小板聚集效果的独立变量,但可能会影响替格瑞洛在体内的转运,而本研究没有测定替格瑞洛的药动学参数,无法确定上述推测,还需进一步研究。试验结果是否会直接影响服用替格瑞洛患者的主要治疗终点,还需对患者进行长期随访。
在高加索人群中进行的全基因组关联分析表明rs4149056与rs113681054存在连锁不平衡(LD)(r=0.8718)致使OATP1B1的转运活性降低,继而使替格瑞洛以及其活性产物血浆水平升高,但是对替格瑞洛治疗期的疗效或安全性没有显著影响[8]。然而,千人基因组计划数据库显示在103名汉族受试者rs4149056与rs113681054表现出较低程度的LD(r = 0.3808),并且rs113681054以及rs4149056这两个SNP对中国健康年轻男性志愿者替格瑞洛的药动学以及药效学(PK/PD)没有显著影响[9]。虽然本研究显示rs4149056 TT型替格瑞洛抗血小板效果较CT型差,但是不具有显著性差别。
目前没有文献报道rs2291075单核苷酸多态性会影响替格瑞洛的转运。本研究也显示rs2291075各基因型对血小板聚集率没有影响。
综上所述,替格瑞洛的抗血小板效果不受相关基因单核苷酸多态性的影响,这为不宜使用氯吡格雷的基因缺陷患者选择替格瑞洛治疗提供了参考。
Effect of pharmacogenetic polymorphism on the antiplatelet aggregation effect of ticagrelor
doi: 10.12206/j.issn.2097-2024.202207087
- Received Date: 2022-07-25
- Rev Recd Date: 2023-03-22
- Available Online: 2023-10-23
- Publish Date: 2023-10-25
-
Key words:
- acute coronary syndrome /
- ticagrelor /
- pharmacogenetic polymorphisms /
- antiplatelet aggregation
Abstract:
| Citation: | XIE Xiaoyun, HUANG Aiwen, LI Li, JIANG Yan, CAI Jiasong. Effect of pharmacogenetic polymorphism on the antiplatelet aggregation effect of ticagrelor[J]. Journal of Pharmaceutical Practice and Service, 2023, 41(10): 629-633, 642. doi: 10.12206/j.issn.2097-2024.202207087 |








 DownLoad:
DownLoad: