-
陈香露白露片的处方由陈皮、甘草、川木香、大黄、石菖蒲五味中药和碳酸镁、氧化镁、次硝酸铋、碳酸氢钠4种化学药组成[1]。方中陈皮行脾胃之气[2,3],为君药,主要活性成分为橙皮苷、川陈皮素、橘皮素,与甘草配伍,健脾祛湿[4];甘草苷、甘草酸铵为甘草的主要活性成分;川木香具行气止痛之效[5],广泛用于腹痛,食欲不振等,木香烃内酯、去氢木香内酯为其主要活性成分。加之碳酸氢钠等碱性药物中和胃酸[6],共成健胃和中、理气止痛功效。
陈香露白露片目前执行的质量标准为《卫生部药品标准WS3-B-1553-93》,该标准仅设置了化学药成分的质量控制参数,难以全面反映产品质量,存在较大的安全风险。为实现陈香露白露片内在质量的有效控制,本实验构建了同步测定陈香露白露片中甘草苷、甘草酸铵、橙皮苷、橘皮素、川陈皮素、木香烃内酯、去氢木香内酯等7种特征活性成分含量的HPLC方法。该研究不仅为提高该制剂质量提供了有效、可靠的分析方法,同时对药品安全风险评估的有效实施也具有重要的研究意义。
-
1260高效液相色谱仪(安捷伦公司),配置DAD检测器;XS105DU电子天平、ME 204E电子天平(梅特勒-托利多公司);KQ-300DE数控超声仪(昆山超声仪器有限公司)。
-
对照品甘草苷(批号:111610-201908,含量95.0%)、甘草酸铵(批号:110731-202021,含量96.2%)、橙皮苷(批号:110721-202019,含量95.3%)、川陈皮素(批号:112055-202001,含量99.6%)、橘皮素(批号:112054-202001,含量99.8%)、木香烃内酯(批号:111524-201911,含量99.9%)、去氢木香内酯(批号:111525-201912,含量99.5%)均购于中国食品药品检定研究院;甲醇、乙腈(色谱纯,赛默飞公司);其余试剂均为分析纯(购于国药集团化学试剂有限公司);实验用水为娃哈哈纯净水。16批陈香露白露片样品来自3个生产厂家:广西圣特药业有限公司(企业1),批号201207(S1)、190202(S2)、200506(S3)、210106(S4)、210107(S5)、210110(S6)、201102(S7);贵州百灵企业集团制药有限责任公司(企业2),批号20191211(S8)、20210105(S9)、20210111(S10)、20201212(S11)、20201001(S12);四川康福来药业集团(企业3),批号200902(S13)、200901(S14)、201202(S15)、200601(S16),以上样品规格均为每片重0.3 g。
-
采用ZORBAX Eclipse XDB-C18色谱柱(4.6 mm×250 mm,5 μm),流动相A为甲醇,流动相B为0.1%磷酸水溶液,梯度洗脱(0~8 min,15% A;8~35 min,15% A→50% A;35~50 min,50% A;50~65 min,50% A→80% A;65~66 min,80% A→15% A;66~75 min,15% A),流速1.0 ml/min,柱温35 ℃,进样量10 μl,检测波长237 nm(0~15 min,检测甘草苷)、283 nm(15~25 min,检测橙皮苷)、237 nm(25~34 min,检测甘草酸铵)、330 nm(34~45 min,检测川陈皮素、橘皮素)、237 nm(45~75 min,检测去氢木香内酯、木香烃内酯)。
-
取甘草苷、橙皮苷、甘草酸铵、川陈皮素、橘皮素、木香烃内酯、去氢木香内酯对照品适量,精密称定,用50%甲醇配制成浓度分别为55.72、1108、307.2、56.25、18.75、26.01、58.95 μg/ml的混合对照溶液。
-
取陈香露白露片样品,研细,精密称取约1 g,置于锥形瓶中,精密加入25 ml 50%甲醇,超声30 min(功率250 W,频率33 kHz),放冷,精密称定,用50%甲醇补充损失的重量,摇匀,0.45 μm微孔滤膜滤过,取续滤液,即得。
-
分别按陈香露白露片处方中各药比例制备缺陈皮、甘草、川木香的阴性样品,并按“2.2.2”项下方法制成缺陈皮、甘草、川木香的阴性供试品溶液。
-
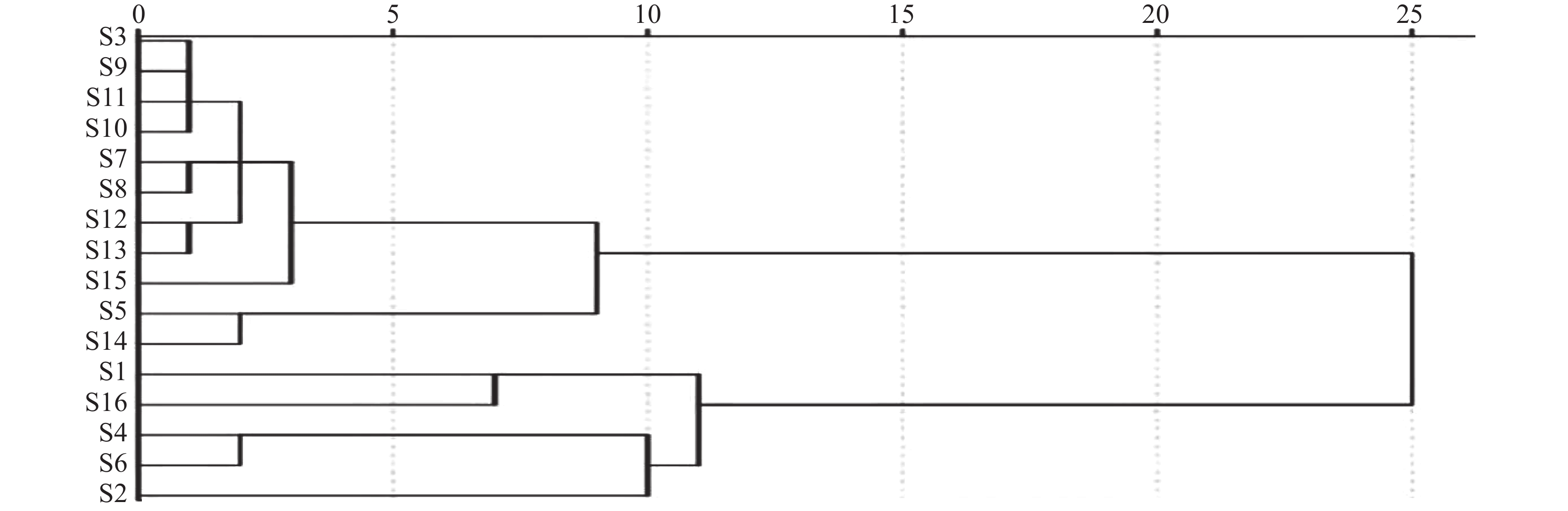
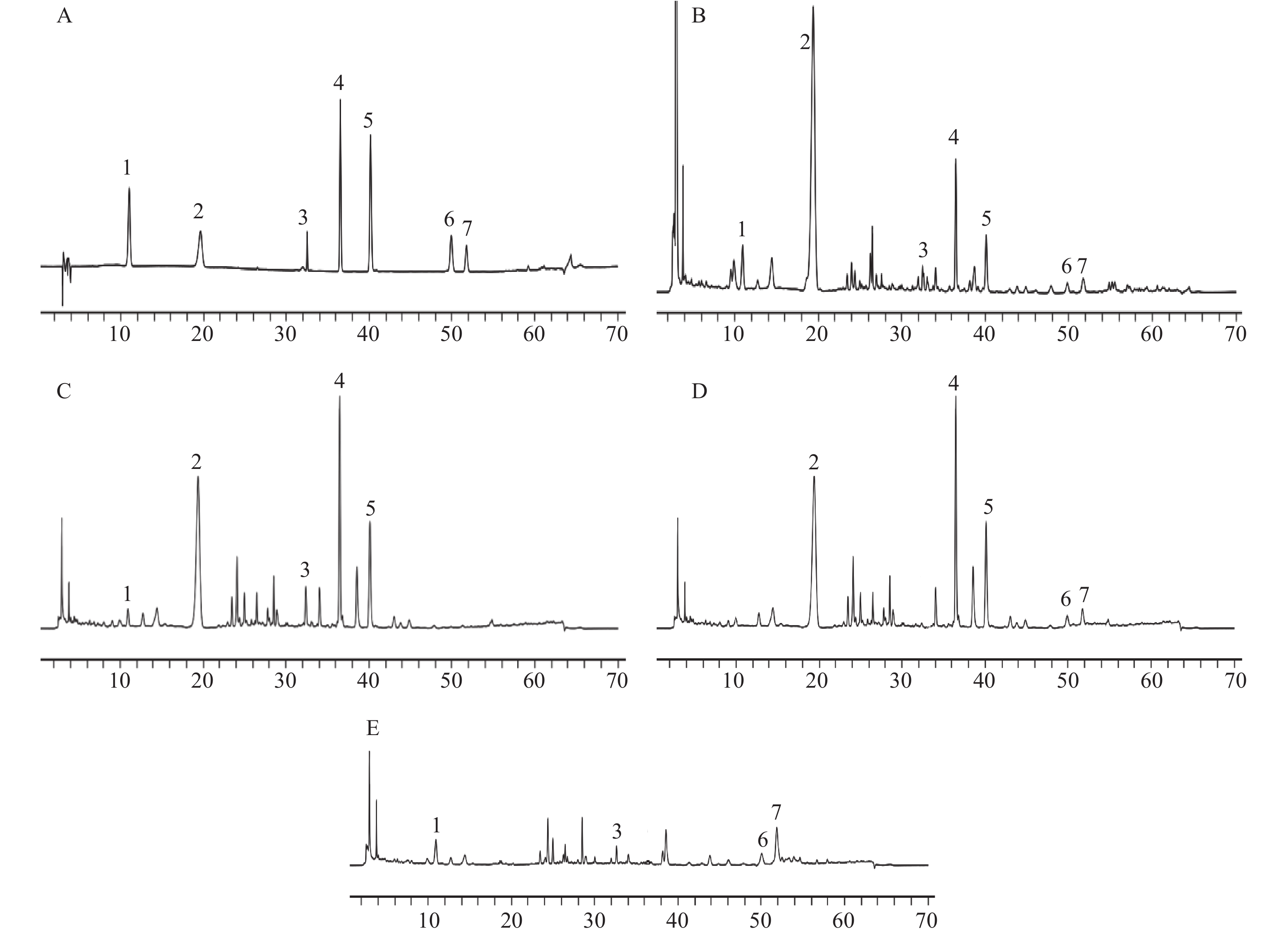
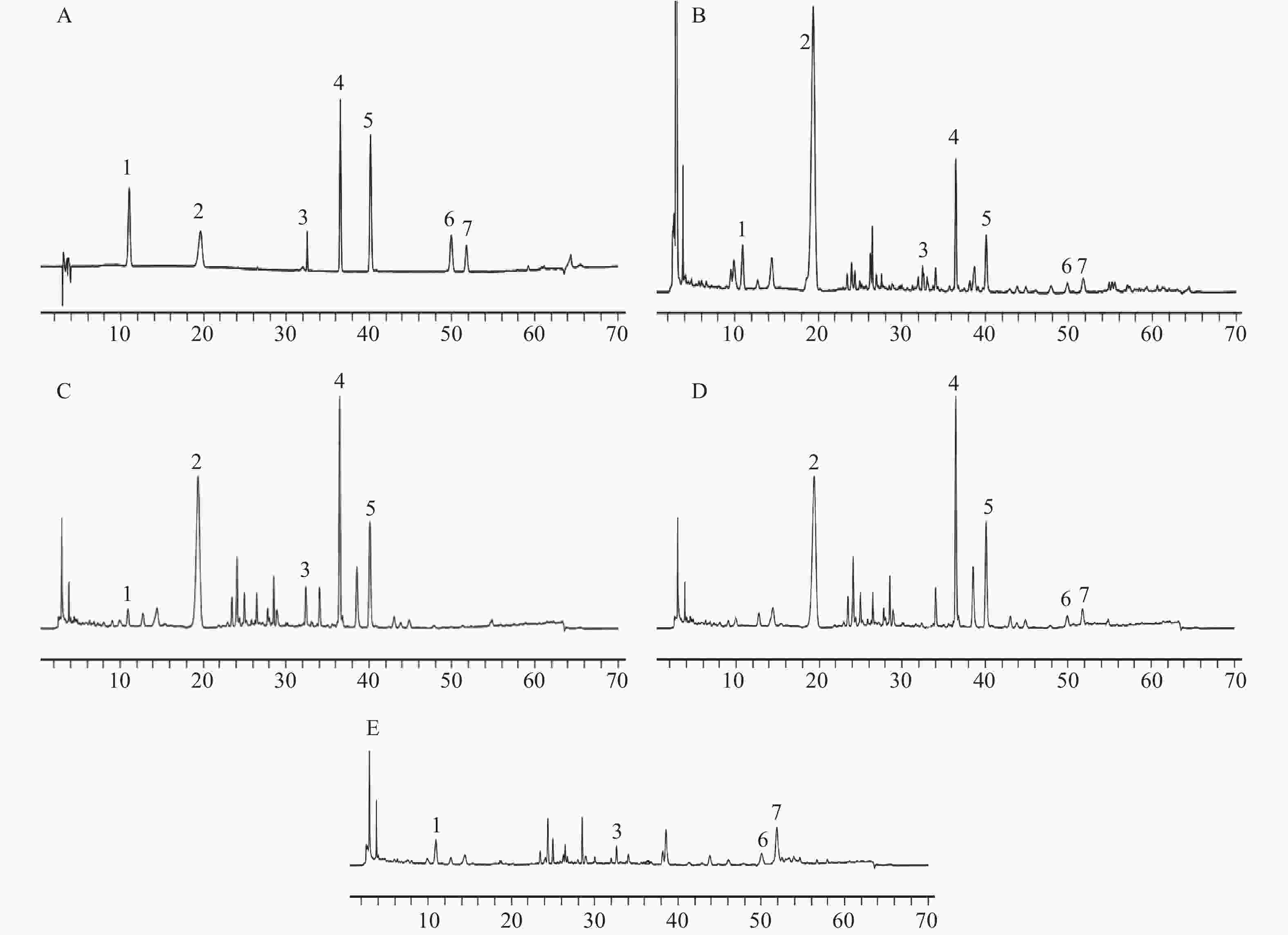
对照品溶液、供试品溶液及缺陈皮、甘草、川木香的阴性供试品溶液按“2.1”项下色谱条件进行测定分析。结果如图1所示,甘草苷、甘草酸铵、橙皮苷、川陈皮素、橘皮素、木香烃内酯、去氢木香内酯等7种成分与相邻峰分离良好,分离度均大于1.5;阴性样品中其他成分色谱峰均未对7种成分产生干扰,表明该分析方法专属性良好。
-
精密吸取“2.2.1”项下对照品溶液0.10、0.50、1.00、2.00、3.00、5.00 ml置于5 ml容量瓶中,用50%甲醇稀释,制备6个浓度的对照品溶液。按“2.1”项下色谱条件进行测定,并绘制标准曲线,其中横坐标为对照品的浓度(X,μg/ml),纵坐标为峰面积(Y)。7种成分线性范围、线性回归方程与相关系数见表1。结果显示,7种成分分别在各自浓度范围内线性关系良好。
成分 线性范围(μg/ml) 回归方程 r 甘草苷 1.110~55.72 Y=17125X+155.53 0.9992 橙皮苷 22.15~1108 Y =5784.3X+7.1165 0.9995 甘草酸铵 6.140~307.2 Y =15716X+375.13 0.9999 川陈皮素 1.130~56.25 Y =21135X+32.117 0.9997 橘皮素 0.3700~18.75 Y =20148X+72.316 0.9982 木香烃内酯 0.5200~26.01 Y=21432X+4.1189 0.9991 去氢木香内酯 1.180~58.95 Y=15235X-135.15 0.9999 -
取“2.2.1”项下对照品溶液,按“2.1”项下色谱条件重复测定6次。结果显示甘草苷、甘草酸铵、橙皮苷、川陈皮素、橘皮素、木香烃内酯、去氢木香内酯峰面积6次测定结果的RSD分别为0.37%、0.38%、0.39%、0.42%、0.48%、0.75%、0.67%,表明该仪器精密度良好。
-
按“2.2.2”项下方法平行制备6份陈香露白露片(批号:20210105)供试品溶液,按“2.1”项下色谱条件进行测定。结果显示甘草苷、甘草酸铵、橙皮苷、川陈皮素、橘皮素、木香烃内酯、去氢木香内酯的含量依次分别为1.158、4.002、10.68、0.0633、0.1477、0.1323、0.2927 mg/g,其RSD分别为0.62%、0.65%、0.64%、1.05%、1.62%、0.87%、1.28%,表明该方法重复性良好。
-
取同一供试品溶液(批号:20210105),放置 0、4、8、12、16、20、24 h后,按“2.1”项下色谱条件进行测定。结果显示甘草苷、甘草酸铵、橙皮苷、川陈皮素、橘皮素、木香烃内酯、去氢木香内酯峰面积的RSD依次分别为0.54%、0.68%、0.72%、1.02%、0.85%、1.60%、1.32%,表明该供试品溶液在24 h内具有良好的稳定性。
-
取已知含量的陈香露白露片(批号:20210111)9份,每份约0.5 g,精密称定,基于样品中7种成分的含量分别按低、中、高3种浓度水平加入对照品溶液(每个浓度各3份),按“2.2.2”项下方法制备供试品溶液,并按“2.1”项下色谱条件进行测定,结果显示7种成分的回收率分别为98.71%、98.12%、98.44%、98.22%、99.17%、99.18%、97.93%,RSD分别为0.16%、0.67%、0.57%、0.62%、0.48%、0.56%、0.58%,表明该方法准确性高。
-
取16批陈香露白露片样品,按“2.2.2”项下方法制备供试品溶液,并在“2.1”项下色谱条件测定样品。外标法计算16批次样品中7种成分的含量,结果见表2。结果显示16批样品中甘草苷、甘草酸铵、橙皮苷、川陈皮素、橘皮素、木香烃内酯、去氢木香内酯的含量分别为0.1250~1.174、2.354~7.426、1.822~27.21、0.0370~1.399、0.0723~0.4433、0.0140~0.1990、0.2207~1.407 mg/g。
批号 甘草苷 橙皮苷 甘草酸铵 川陈皮素 橘皮素 木香烃内酯 去氢木香内酯 201207 0.5967 1.822 2.354 0.0847 0.1840 0.1990 0.9440 190202 0.6090 3.513 3.413 1.399 0.2773 0.0487 1.407 200506 1.053 9.611 3.643 0.351 0.0723 0.0140 0.4123 210106 0.6203 3.634 2.682 0.0433 0.0930 0.0673 0.2647 210107 0.6433 3.855 2.761 0.0480 0.2883 0.0460 0.2207 210110 0.2047 3.034 2.689 0.3313 0.1237 0.0993 0.2907 201102 0.6677 5.209 2.613 0.1530 0.2897 0.0417 0.4200 20191211 1.107 6.832 3.724 0.4360 0.0843 0.0180 0.2243 20210105 1.158 10.68 4.002 0.0633 0.1477 0.1323 0.2927 20210111 1.174 9.624 3.971 0.0370 0.0830 0.1857 0.8773 20201212 1.085 9.204 3.860 0.0993 0.2333 0.1890 0.4257 20201001 1.055 10.333 3.682 0.8917 0.1937 0.1023 1.293 200902 0.747 8.085 2.947 0.9430 0.2137 0.0870 1.326 200901 0.8807 27.21 3.080 1.044 0.2270 0.1263 0.5503 201202 0.5473 5.573 2.584 1.144 0.2437 0.1280 0.3333 200601 0.1250 5.374 7.426 0.0883 0.4433 0.0650 0.3890 -
本实验色谱条件参考《中华人民共和国药典》(2020年版)一部,237 nm为甘草苷、甘草酸铵的检测波长[7],225 nm为木香烃内酯、去氢木香内酯的检测波长[7],对两个检测波长均进行了待测成分响应值及专属性的考察,结果显示各成分在237 nm波长处响应值均较高且与杂质峰分离良好,故最终选择237 nm作为甘草苷、甘草酸铵、木香烃内酯、去氢木香内酯等4种成分的检测波长。而橙皮苷、川陈皮素、橘皮素均参考药典上的检测波长[7]。陈香露白露片中所含成分复杂,等度洗脱很难将7个化学成分同时分离,故采用梯度洗脱。
-
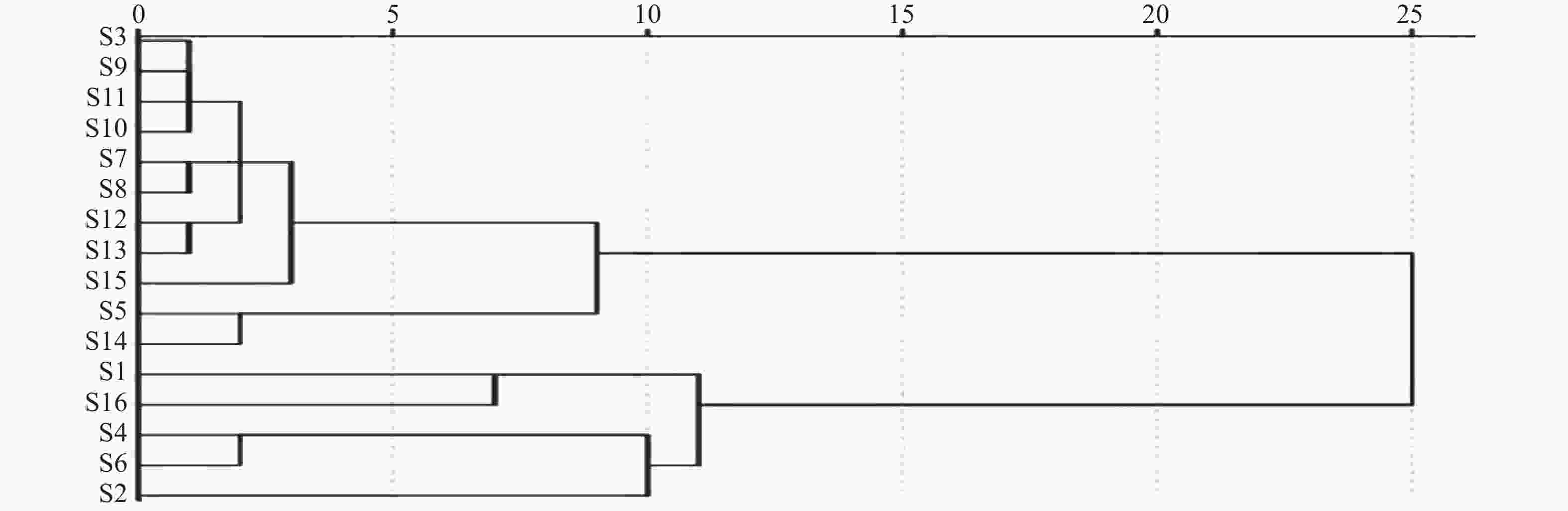
利用SPSS软件以16批陈香露白露片中7种有效成分含量作为变量进行聚类分析。结果如图2所示,16批样品以度量值11为阈值,聚分为2大 类,其中一大类主要为企业2和3的样品,另一大类主要为企业1的样品。结果表明由于企业缺少对该品种中重要指标成分的含量控制,导致产品在不同企业,以及相同企业不同批次之间质量差异较大。
-
由表2得知,16批陈香露白露片7种主要成分含量差异较大。其中,橙皮苷含量差异主要表现在不同生产企业之间,主要原因可能是各生产企业使用了不同产地、不同采收期、不同生长年限的原药材导致的差异。而去氢木香内酯含量差异主要表现在同一生产企业的不同批次之间,其原因可能是药材养护措施不当、生产工艺控制参数不合理导致挥发性成分去氢木香内酯损失产生的差异。基于上述研究,建议生产企业加强对陈皮、甘草、川木香等原药材中关键活性成分的质量控制,建立高于《中华人民共和国药典》的内控标准,严格按批准的处方和生产工艺进行投料与生产,进一步提高陈香露白露片的质量一致性,从而实现其疗效的稳定性和有效性。
Simultaneous determination of seven constituents in Chenxiang Lubailu tablet by HPLC
doi: 10.12206/j.issn.2097-2024.202202038
- Received Date: 2022-02-16
- Rev Recd Date: 2022-05-30
- Available Online: 2022-11-28
- Publish Date: 2022-11-25
-
Key words:
- HPLC /
- Chenxiang Lubailu tablet /
- liquiritin /
- ammonium glycyrrhizinate /
- hesperidin /
- nobiletin /
- tangeretin /
- costunolide /
- dehydrocostuslactone
Abstract:
| Citation: | ZHANG Lu, DAI Qunfang, CHEN Sisi. Simultaneous determination of seven constituents in Chenxiang Lubailu tablet by HPLC[J]. Journal of Pharmaceutical Practice and Service, 2022, 40(6): 553-556, 570. doi: 10.12206/j.issn.2097-2024.202202038 |







 DownLoad:
DownLoad: