-
阿弗他溃疡(RAU)又称为复发性口腔溃疡,其发病率高,约占口腔黏膜病的20%[1],发病人群主要是青壮年,女性居多,春、冬季发病率较高[2]。表现为口腔黏膜反复发作的溃疡,呈假膜覆盖的凹陷面,中间为白色炎症,边缘红肿。目前病因病机尚不明确[3],被广泛接受的诱发因素有气候环境、食物营养、心理精神和免疫因素等[4],研究发现口腔溃疡的发病可能与口腔微生物密切相关[5],可通过纠正口腔菌群平衡达到加速愈合、控制炎症、抑制复发。经过前期研究,选用硫酸新霉素,针对口腔菌群中异常增多的G−菌;克霉唑对引起口腔黏膜疾病的白念珠菌针对性强;选用不发生双硫仑反应的奥硝唑作为抗厌氧菌药物;达克罗宁作为局麻药,黏膜穿透性强,局麻时间长;采用冰片作为创口收敛剂,兼具抗炎、消肿、改善气味的功能;甘草次酸作为抗炎剂,与糖皮质激素相比长期使用副作用较低。以上6种成分之间无配伍禁忌,其中硫酸新霉素和克霉唑口服均不吸收,安全性高[4]。采用物理凝聚法分散水不溶性成分,通过溶剂浇铸法制备膜剂[6-9]。本课题采用层次分析法结合单因素考察对不同成膜材料的黏附时间、溶蚀时间等9项考察指标综合评分,优选出最合适的成膜材料;采用层次分析与正交试验相结合,优选成膜材料的最佳配比,兼顾使用舒适性和生产适用性的同时,重点筛选出黏附性好且缓释时间较长的成膜材料配比。
-
AE240精密电子天平(瑞士METTLER公司);磁力搅拌器(宁波市鄞州群安实验仪器有限公司);托盘天平(福州天平仪器厂);DHG-9145A电热恒温鼓风干燥箱(上海一恒科学仪器有限公司);水浴恒温振荡器(上海智城分析仪器制造有限公司);OS2O-Pro型搅拌器(北京大龙试验仪器有限公司);拉力测试仪(和晟仪器科技有限公司);游标卡尺(上海台海工量具有限公司)。
-
聚乙烯醇1788(上海阿拉丁生化科技股份有限公司,批号:20170318);羧甲基纤维素钠(国药集团化学试剂有限公司,批号:20180412);海藻酸钠(国药集团化学试剂有限公司,批号:20190810);羟丙基纤维素(上海阿拉丁生化科技股份有限公司,批号:20170415);羟丙甲基纤维素钠(上海阿拉丁生化科技股份有限公司,批号:20170318);明胶(上海阿拉丁生化科技股份有限公司,批号:20180517);水胶体敷料(康维德医疗用品有限公司,批号:9F04020)。硫酸新霉素(南京都莱生物技术有限公司,批号:20180312);克霉唑(上海阿拉丁生化科技股份有限公司,批号:20170518);奥硝唑(上海阿拉丁生化科技股份有限公司,批号:20180522);盐酸达克罗宁(苏州裕元生物科技有限公司,批号:20170215);甘草次酸(天津希恩思生化科技有限公司);冰片(亳州寿言斋健康科技有限公司,批号:20191021)。
-
取聚乙烯醇1788(PVA-1788)、羟丙基纤维素(HPC)、羟丙甲基纤维素(HPMC)、甲基纤维素(MC)、海藻酸钠(SA)、羧甲基纤维素钠(CMC-Na)、明胶(GEL)各3 g置于60 ml水中搅拌1 h,然后加热至60 ℃,搅拌至完全溶解得成膜材料凝胶,采用溶剂浇铸法铺展于培养皿中,置60 ℃烘箱烘干。从外观、拉伸性能、厚度、成膜时间、脱模效果、溶胀系数、溶蚀时间、黏附力和黏附时间9个方面进行评价。
-
膜剂成品外观评价标准:①气泡:无气泡得5分;少量气泡,易除去,得4分;少量气泡,经1 h静置可除去,得3分;需大于1 h静置、抽真空、离心等方法才能将气泡除去,得2分;采用各种方法处理后膜剂中仍存在大量气泡得1分;气泡较多,干燥后无法成完整膜剂得0分。②颜色:无色透明得5分;半透明得4分;半透明且带有黄色或其他较浅颜色得3分;半透明且有絮状、颗粒状纹路得2分;呈不透明颜色,且有絮状、颗粒状纹路得1分;外观颜色较深、纹理较多、不透明得0分。③柔软度:柔软、不卷曲得5分;柔软稍有卷曲得4分;反向卷曲后缓慢恢复原来形状得3分;膜较硬,卷曲后快速恢复原来形状得2分;卷曲可能碎裂,得1分;硬度较大无法制得膜剂得0分。从表1和图1可知,PVA-1788和HPC颜色透明,气泡较少,柔软度较好,外观较其他材料好。
成膜材料 气泡 颜色 柔软度 总评分
(分)性状 评分
(分)性状 评分
(分)性状 评分
(分)PVA-1788 无 5 透明 5 很柔软 5 15 HPC 无 5 透明 5 很柔软 5 15 HPMC 无 5 透明 5 厚、硬 2 12 MC 大量 2 白色 4 较硬 3 9 SA 少量 4 黄色 2 厚、硬 2 8 CMC-Na 少量 4 白色 5 很柔软 5 14 GEL 无 5 淡黄色 4 厚、硬 2 11 注:PVA-1788:聚乙烯醇1788;HPC:羟丙基纤维素;HPMC:羟丙甲基纤维素;MC:甲基纤维素;SA:海藻酸钠;CMC-Na:羧甲基纤维素钠;GEL:明胶 -
取各实验组膜剂1片(2 cm×1 cm),利用拉力测试仪测量各实验组膜剂的拉伸长度与断点力,通过拉伸长度/断点力的比值判断拉伸性能,数值越大,可拉伸距离越大,膜剂拉断所需力相对越小,与口腔黏膜贴敷时顺应性越高。以拉伸长度/断点力比值的最大值作为100%,采用归一化法对其他各组进行评价,各实验组测定3次求平均值。结果见表2,PVA-1788的拉伸性能明显优于其他材料。
成膜材料 拉伸长度
(l/mm)断点力
(f/kg)拉伸长度/
断点力评分
(分)PVA-1788 24.24 2.137 11.34 100 HPC 4.935 1.644 3.002 26.47 HPMC 0.925 4.063 0.227 0.020 MC 2.180 7.443 0.293 0.026 SA 2.638 4.566 0.578 0.051 CMC-Na 2.611 7.772 0.336 0.030 GEL 0.436 2.056 0.212 0.019 -
分别从各实验组中选取10片膜剂,用游标卡尺测量总厚度,计算每片膜剂的平均厚度,取平均厚度的倒数,以最大值作为100%,用归一化法对其他各实验组膜剂进行评分。由表3可知,制备的膜剂中,HPC厚度最薄,MC最厚,其余各组差异较小,膜剂越薄口腔黏膜舒适性越好。
成膜材料 膜剂厚度 成膜时间 脱膜效果 测定值
(l/mm)评分
(分)时间
(t/min)评分
(分)面积
(s/cm2)评分
(分)PVA-1788 0.13 76.92 240 60.0 54.0 100 HPC 0.10 100.00 330 42.9 43.2 80 HPMC 0.11 90.91 143 100.0 54.0 100 MC 0.15 66.67 258 55.7 54.0 100 SA 0.11 90.91 195 72.9 54.0 100 CMC-Na 0.12 83.33 255 55.7 54.0 100 GEL 0.12 83.33 238 60.0 27.0 50 -
对各实验组的成膜时间进行测定,取成膜时间的倒数,以倒数的最大值为100%,用归一化法对其他成膜材料进行评分,成膜时间短,有利于提高生产效率,结果见表3。
-
以剥离最大面积的膜的面积占培养皿(半径4.3 cm)的百分比作为评判标准,以完全脱模为100%。结果见表3,PVA-1788、HPMC、MC、SA、CMC-Na可以直接撕取完整膜剂,脱模较容易。
-
称取0.4 g氯化钠、0.795 g氯化钙、0.4 g氯化钾、1.0 g尿素、0.78 g磷酸钠、0.005 g硫化钠,加热水400 ml溶解,放冷后转移至1000 ml容量瓶中,用纯化水稀释至刻度,用氢氧化钠调节pH至6.8,即得人工唾液。取各试验组膜剂1片(1 cm×1 cm),称量记为m,将膜剂放入培养皿中称重记为W0。将膜剂润湿黏附于培养皿中,加入人工唾液[10-11],分别在10、30、60、120、240 min时,倒去人工唾液,称量培养皿加膜剂的质量Wi。计算公式如下:溶胀系数=(Wi−W0)/m×100%;每组平行3次,求各时间点溶胀系数的平均值,以各组溶胀系数最大的值作为100%,用归一化法对其他各组进行评分。由表4中结果可知,CMC-Na溶胀系数最大。
成膜材料 溶胀系数(%) 评分
(分)10 min 30 min 60 min 120 min 240 min 480 min PVA-1788 396 429 549* 312 0 — 18.35 HPC 731 936* 806 447 0 — 31.28 HPMC 641 1028* 579 10 0 — 34.36 MC 811 884 1170 1456* 1660 1528 55.48 SA 1489 2468* 2232 283 0 — 82.49 CMC-Na 1023 1670 2262 2849 2992* 2357 100 GEL 622 548 553 759* 627 0 25.37 注:*表示各试验组最大溶胀系数,—表示溶蚀殆尽,实验终止。 -
取1片膜剂(1 cm×1 cm)称重记为m,称取2 ml EP管称重记为n。将膜剂润湿黏贴在EP管下缘。在EP管中加入2 ml人工唾液,在37 ℃恒温振荡器中以100 r/min振荡10 min,倒出液体,称重记为W0,然后重新加入2 ml纯化水并恒温振荡20 min,如此重复10次,于各时间点倒出管内液体后称重,依次记为Wi。以相邻时间点重量差异计算溶蚀速率,公式如下:溶蚀速率=[(Wi−Wi−1)/20(W0−m−n)]×100%。以各组溶蚀时间最大值作为100%,用归一化法对其他各组进行评分。结果见表5,MC溶蚀时间较长,在200 min内未出现明显溶蚀减重,溶蚀系数出现负数是由于溶胀增重略大于溶蚀损失,这将阻碍药效成分随着膜剂溶蚀的释放。CMC-Na溶蚀速率较小,其溶蚀时间可长达180 min,缓释性能显著优于其他成膜材料,较适合制备缓释膜剂。
成膜材料 连续时间点的平均溶蚀百分率(%) 溶蚀时间
(t/min)每分钟溶
蚀速率(%)评分
(分)1 2 3 4 5 6 7 8 9 10 PVA-1788 24.62 36.92 20.00 18.46 0.00 — — — — — 80 1.25 40 HPC 37.50 24.04 38.46 0.00 — — — — — — 60 1.67 30 HPMC 54.55 45.45 0.00 — — — — — — — 40 2.5 20 MC 0.00 −12.26 0.94 −6.60 −2.83 0.00 −3.77 6.60 −3.77 7.55 200 −0.07 100 SA 49.07 50.93 0.00 — — — — — — — 40 2.5 20 CMC-Na −17.71 −2.21 4.79 15.46 26.19 37.14 20.88 14.20 1.26 0.00 180 0.56 90 GEL 100.0 0.00 — — — — — — — — 20 5.0 10 注:“—”代表溶蚀殆尽,实验终止。 -
黏附膜剂黏附力测定[10]:取两块同样大小的橡皮,分别固定于天平托盘上及玻璃板下方,调节高度和重量使上下橡皮相互接触时天平平衡。取膜剂(1 cm×1 cm)预先用人工唾液润湿,黏于下橡皮表面,将上橡皮与膜剂接触,天平空托盘加50 g砝码使上下橡皮自然按压膜剂90 s,移去50 g砝码,在下橡皮固定的托盘里依次加入重量逐渐增加的砝码,以5 s内上、下橡皮分离为标准,所加砝码的重量即为黏附力。将各组黏附力最大值作为100%,用归一化法对其他各组进行评分。由表6可知,MC的黏附力最好,其次是PVA-1788。
成膜材料 黏附力 黏附时间 称重
(m/g)评分
(分)时间
(t/min)评分
(分)PVA-1788 121 88.97 100 100 HPC 100 73.53 60 60 HPMC 40 29.41 50 50 MC 136 100 85 85 SA 119 87.5 80 80 CMC-Na 90 66.18 38 38 GEL 6.7 0.05 5 5 -
取水胶体敷料(2 cm×2 cm)粘贴于500 ml烧杯内侧,用人工唾液润湿膜剂(1 cm×1 cm),将膜剂按压在水胶体敷料上约20 s,在膜剂表面覆盖一层1 cm×1 cm薄塑料膜用以降低溶蚀对黏附时间的干扰,在烧杯中加入人工唾液,液面没过膜剂。在恒温37 ℃,150 r/min搅拌,于300 min内监测黏附时间[12],各组试验组取3片计算平均值。将各组黏附时间的最大值作为100%,用归一化法对其他各组进行评分。由表6可知,PVA-1788的黏附时间最长,可达100 min。
-
根据“2.1”项下9个考察指标对缓释膜剂的贡献度,设计两两比较矩阵,见表7。
指标 外观 厚度 拉伸
性能脱模
效果成膜
时间黏附
时间黏附
力溶胀
系数溶蚀
速度外观 1 1 1 1 1 0.5 0.5 0.33 0.25 厚度 1 1 1 1 1 0.5 0.5 0.33 0.25 拉伸性能 1 1 1 1 1 0.5 0.5 0.33 0.25 脱模效果 1 1 1 1 1 0.5 0.5 0.33 0.25 成膜时间 1 1 1 1 1 0.5 0.5 0.33 0.25 黏附时间 2 2 2 2 2 1 1 0.5 0.5 黏附力 2 2 2 2 2 1 1 0.5 0.5 溶胀系数 3 3 3 3 3 2 2 1 1 溶蚀速度 4 4 4 4 4 2 2 1 1 -
根据各指标对比的优先矩阵(表3)及公式(1)计算初始权重系数Wi′:
式中m为受检验层次目标数,a1~am为矩阵两两比较的评分,经计算各指标成分初始权重系数分别为:W1′=0.65042、W2′=0.65042、W3′=0.65042、W4′=0.65042、W5′=0.65042、W6′=1.25992、W7′=1.25992、W8′=2.14765、W9′=2.51984。
按照公式(2)计算归一化权重系数Wi,
得各指标成分权重系数W1=0.06230、W2=0.06230、W3=0.06230、W4=0.06230、W5=0.06230、W6=0.12069、W7=0.12069、W8=0.20572、W9=0.24138。
-
CR为随机一致性比率,定义CR=CI/RI作为衡量所得权重系数是否合理的指标,一致性指标CI=(λmax−m)/(m−1),式中m为次级目标数,矩阵的最大特征根
$\lambda_{m=x}=1 / m {\sum\limits_{j=1}^{m}}\left[{\sum\limits_{j=1}^{m}}\left(a_{j j} \times W_{j}\right)+W_{j}\right]$ ,当矩阵阶数=9时,平均随机一致性指标RI=1.45。经计算λmax=9.0153;则CI=(λmax−m)/(m−1)=0.00192;CR=CI/RI=0.00192/1.45=0.00132;CR<0.1则表明9项指标优先比较矩阵满足一致性要求,故所得权重系数有效,结果具有一致性。 -
根据单因素考察所得出的评分(表1~6),结合层次分析法权重系数,对成膜材料性能进行综合评分,其中,CMC-Na和PVA-1788综合评分最高,分别为87.45和64.49。详见表8。
成膜材料 外观 厚度 拉伸性能 成膜时间 脱模效果 黏附时间 黏附力 溶胀系数 溶蚀速度 评分
(分)PVA-1788 6.23 4.79 6.23 3.70 6.23 12.10 10.74 3.77 10.73 64.49 HPC 6.23 6.23 1.65 2.69 4.98 7.24 8.87 6.44 8.05 52.38 HPMC 4.98 5.66 0.125 6.23 6.23 5.98 3.55 7.07 5.36 45.19 MC 3.74 4.15 0.161 3.45 6.23 9.20 10.56 11.42 0.00 48.90 SA 3.32 5.66 0.317 4.55 6.23 4.37 7.99 16.97 5.36 54.78 CMC-Na 5.82 5.19 0.185 3.48 6.23 9.77 12.07 20.57 24.14 87.45 GEL 4.57 5.19 0.117 3.74 3.12 0.57 0.595 5.222 2.68 25.80 通过单因素考察各种成膜材料,筛选出CMC-Na和PVA-1788,两种膜剂基质各有优点。CMC-Na溶胀系数大、溶蚀时间长,可改善其他基质较快溶解的缺点。PVA-1788黏附力大、黏附时间长,是良好的黏附性材料,可改善膜剂外观和柔软度,提高成膜效率和成品率。
-
采用物理凝聚法结合溶剂浇铸法设计膜剂制备工艺如下:取1.56 g的PVA-1788加入12.5 ml水,以300 r/min搅拌30 min,后加热至60 ℃搅拌至完全溶解,得PVA-1788凝胶。取4.68 g的CMC-Na加入117 ml水,以800 r/min在45 ℃下搅拌至完全溶解,得CMC-Na凝胶。将两种凝胶搅拌混合均匀,作为溶液①;分别取硫酸新霉素、盐酸达克罗宁、甘草次酸,分别加入40 ml水溶解,作为溶液②;取克霉唑、奥硝唑与冰片加入30 ml无水乙醇搅拌至溶解,作为溶液③;将溶液②、③缓慢加入45 ℃溶液①中,边加边以1 000 r/min搅拌,得质地均匀的含药混悬凝胶,静置消泡。
取凝胶16 g,浇铸铺展于半径为4.3 cm的培养皿中。取一份立即放置于烘箱中60 ℃干燥5 h;另取一份凝胶放置4 ℃冰箱12 h后转移至烘箱中60 ℃干燥5 h。待完全干燥后,将膜剂分割成2 cm×1 cm 小片,即得口腔膜剂,结果见图2。物理凝聚法制备的凝胶趁热烘干制得膜剂表面为乳白色不透明,颜色均匀。而物理凝聚法制备的凝胶经低温放置12 h制得膜剂烘干后可看到颗粒较小的沉淀,说明在低温下放置时间过长可能形成较大结晶,影响膜剂质量,趁热烘干可抑制水不溶性药物的析出。
-
在筛选出CMC-Na和PVA-1788作为成膜材料的基础上配伍一定比例甘油作为增塑剂,可提高膜剂的柔软度和脱膜效果,用量为成膜材料质量的0.5%~2%。按“2.3”方法制备膜剂进行正交试验,正交试验因素水平表见表9。
水平 A因素PVA-1788
用量(m/g)B因素CMC-Na
用量(m/g)C因素甘油
用量(%)1 1 1 0.5 2 2 2 1 3 3 3 2 -
以外观、厚度、拉伸性能、脱膜效果、成膜时间、溶胀系数、溶蚀速率、黏附时间、黏附力9个方面为评价指标,按照单因素考察的试验方法,结合“2.2.2”项下层次分析法各考察指标的权重系数计算综合评分(表10),通过方差分析(表11)和直观分析(表12)优选膜剂成膜材料的最佳比例。
组别 外观 厚度 拉伸性能 成膜时间 脱模效果 黏附时间 黏附力 溶胀系数 溶蚀速度 综合评分
(分)1 5.40 6.23 1.58 5.19 4.36 12.07 9.06 12.45 17.24 73.58 2 5.40 6.23 3.50 5.19 3.74 12.07 10.56 14.92 17.24 78.85 3 4.98 6.23 2.63 5.56 3.74 4.83 11.31 20.57 24.14 83.99 4 5.82 6.23 6.23 5.99 3.12 3.22 8.30 7.77 20.69 67.37 5 5.40 6.23 2.44 4.58 6.23 12.07 8.30 11.48 24.14 80.87 6 4.15 5.19 0.67 5.66 3.74 12.07 12.07 16.05 20.69 80.29 7 5.82 6.23 4.36 5.77 6.23 12.07 6.03 7.46 20.69 74.66 8 6.23 5.19 0.58 5.37 4.98 12.07 9.05 9.99 20.69 74.16 9 5.39 6.23 0.96 6.23 4.36 12.07 5.28 9.95 24.14 74.61 因素 偏差平方和 自由度 F比 F临界值 P PVA-1788用量(m/g) 0.003 2 3.000 19.000 >0.05 CMC-Na用量(m/g) 0.009 2 9.000 19.000 >0.05 甘油用量(%) 0.006 2 6.000 19.000 >0.05 误差 0.00 2 序号 A因素PVA-
1788用量(m/g)B因素CMC-Na
用量(m/g)C因素甘油
用量(%)综合评分
(分)1 1 1 1 73.58 2 1 2 2 78.85 3 1 3 3 83.99 4 2 1 2 67.37 5 2 2 3 80.87 6 2 3 1 80.29 7 3 1 3 74.66 8 3 2 1 74.159 9 3 3 2 74.61 K1 0.850* 0.781 0.816 K2 0.821 0.838 0.798 K3 0.804 0.855* 0.861* R 0.046 0.074 0.063 注:*表示各因素最优水平条件 从正交试验方差结果显示,PVA-1788用量、CMC-Na用量、甘油用量对综合评分的贡献无显著性差异,三者用量配比需通过直观分析结果进行合理分配,正交试验直观分析显示,CMC-Na用量对综合评分影响较大,其次是甘油的用量,PVA-1788用量对综合评分影响较小。最优条件为A1B3C3,即最佳配比为:PVA-1788与CMC-Na的比例为1∶3,甘油用量为成膜材料用量的2%,与正交试验第3组试验条件一致,综合评分为83.99。
-
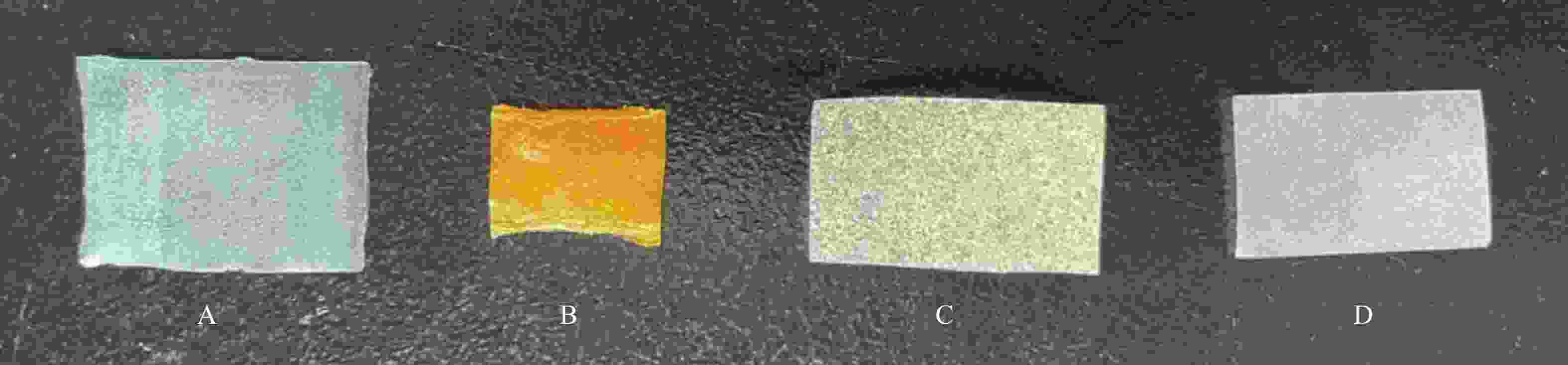
按照“2.4.2”项下最优配比制备口腔溃疡缓释膜剂(D),依次进行外观评价、厚度、拉伸性能、溶胀速率、溶蚀速率、黏附力和黏附时间的测定,方法见“2.1”项下,并与市售复方庆大霉素膜(A)、复方氯己定地塞米松膜(B)及某医院院内制剂(C)进行对比,结果见表13,外观情况见图3。
考察项目 复方庆大霉素膜(A) 复方氯己定地塞米松膜(B) 某医院院内制剂(C) 自制膜剂(D) 气泡 无 无 较多 无 颜色 浅蓝 黄色 浅黄色 白色 柔软度 很柔软 很柔软 较硬 软硬
适中平均拉伸长度(l/mm) 18.844 7.010 0.998 3.443 平均断点力(f/kg) 0.472 1.513 1.190 1.306 平均厚度(l/mm) 0.110 0.120 0.180 0.130 最大溶胀系数(%) 492.5 539.0 1898.1 1939.6 溶蚀时间(t/min) 60.0 60.0 140.0 120.0 黏附力(m/g) 40.0 45.0 5.0 55.0 黏附时间(t/min) 17.3 20.7 53.3 101.7 -
以往对制剂进行研究一般仅以其中1~2种考察指标对制剂的性能进行评价,而考察指标的重要程度则常以主观设定权重,这样评价一种制剂性能的优劣不仅不全面,而且受到主观因素影响较大,往往评价重点考察指标的同时无法兼顾一般的考察指标。正如本课题筛选成膜材料需根据膜剂制备目标综合考察外观、厚度、拉伸性能、成膜时间、脱膜效果、黏附力、黏附时间、溶胀系数和溶蚀时间9个方面的考察指标,面对种类繁多、重要程度各不相同的考察指标,再采用以往的主观评价显得逻辑混乱且无说服力。因此,本课题采用单因素考察结合层次分析法,通过建立指标层不同指标两两比较矩阵,将不同重要性的指标进行统计学处理,得出科学合理的权重系数,以降低主观因素引起的误差,使评价更加全面、合理、客观。通过成膜材料单因素考察可知PVA-1788的弹性、拉伸性能、黏附力和黏附时间优于其他成膜材料,CNC-Na能使得膜剂吸水溶胀逐渐转变成凝胶状,提高膜剂的溶蚀时间。在单因素考察的基础上,设计正交试验,筛选出成膜材料的最佳配比为PVA-1788:CNC-Na为3∶1,并加入两者用量2%的甘油。
物理凝聚法是将分子或离子状态分散的药物溶液加入另一种分散介质中凝聚形成混悬液的方法,可制得10 μm以下的微粒。本试验将不溶于水的克霉唑、奥硝唑和冰片溶于无水乙醇中,在高速搅拌下缓慢加入水溶液中,使药物快速分散成极小的微粒,再利用成膜材料水凝胶的高黏度,抑制结晶增大和沉淀,并采用溶剂浇铸法迅速铺展和干燥,在药物微粒在尚未形成较大结晶前即完成膜剂的干燥,使不溶性药物均匀嵌入膜剂中。
自制膜剂为白色,外观均匀,无气泡,弹性适中,质地柔软,厚度较薄,气味清香,口味微甜,患者顺应性高,在口腔内使用舒适性好。市售复方氯己定地塞米松膜色素含量过高,溶蚀较快,使用后口感较差,对口腔有严重染色现象。市售复方庆大霉素膜具有较大的柔软度和拉伸长度,在实际使用中发现过于柔软,在口腔中易发生皱缩和折叠,在口腔粘贴过程中失败率较高。此外市售两种膜剂的黏附时间和溶蚀时间均较短,实际使用时无法起到长效缓释的作用。某医院院内制剂成膜材料为CMC-Na,因此具有较大的溶胀系数和较长的溶蚀时间,具有良好的缓释作用。单纯使用CMC-Na也存在一些缺陷,如气泡较多、拉伸性能较差、厚度较大、质地较硬等,其中最大的缺陷是CMC-Na初始黏附性较差,在试验过程中初始贴敷成功率较低,极易掉落,需要较长时间吸水溶胀形成凝胶后,才具有一定黏附性。本试验自制膜剂采用PVA-1788与CMC-Na配伍,使膜剂同时具备了较长的黏附时间和溶蚀时间,从黏附和缓释两方面确保膜剂对溃疡创面的滞留,改善了膜剂的外观、柔软度和拉伸性能,起到长效物理隔离和治疗作用。
Study on preparation technology of long-acting sustained-release oral ulcer membrane based on analytic hierarchy process and orthogonal design
doi: 10.12206/j.issn.2097-2024.202109069
- Received Date: 2021-09-13
- Rev Recd Date: 2022-07-24
- Available Online: 2023-08-18
- Publish Date: 2023-08-25
-
Key words:
- aphthous ulcer /
- membrane-forming material /
- analytic hierarchy process /
- orthogonal experiment
Abstract:
| Citation: | XU Xiang, CHEN Xu, KE Yuejiao, LIU Zhihong, CHEN Yufang, ZHOU Xin, SONG Hongtao. Study on preparation technology of long-acting sustained-release oral ulcer membrane based on analytic hierarchy process and orthogonal design[J]. Journal of Pharmaceutical Practice and Service, 2023, 41(8): 501-508. doi: 10.12206/j.issn.2097-2024.202109069 |











 DownLoad:
DownLoad:

