-
亚洲国家儿童和青少年近视罹患率逐年攀升,严重影响到身心健康,并且随着近视程度的加深,还可能出现不可逆的眼底改变,严重时可致盲[1-2]。阿托品是治疗近视的首选药物,广泛运用于临床。1%阿托品临床疗效确切,能有效预防和治疗近视,然而畏光、刺激、干眼等副反应发生率较高,多数患者长期使用时难以耐受[3-7]。多项临床研究对不同浓度阿托品的治疗作用及副作用深入观察,发现0.01%阿托品治疗近视时副作用明显低于1%阿托品,且停药后的反弹现象发生率也显著降低,但在近视快速进展期的控制效果却不及1%阿托品[8-11]。
因此,积极探索合理的治疗方案,在降低阿托品用量的同时保证其治疗效果成为近视治疗的关键和难点。本研究采用豚鼠近视模型观察比较优化方案(交替使用1%、0.01%阿托品)与单一浓度方案在近视控制效果上的差异。
-
选择21 d已断乳的英国三色豚鼠(丹阳市吕城镇奥臣实验养殖场),经眼健康和屈光度筛选(屈光度为2.0~7.0 D,双眼屈光参差<2.0 D,双眼瞳孔直径差值<0.1 mm)后入组。
46只豚鼠随机选取6只作为空白对照组,其余40只随机分为5个干预组:1%阿托品组、0.01%阿托品组、优化组1(1~2周使用1%阿托品,3~4周使用0.01%阿托品)、优化组2(1~2周0.01%阿托品,3~4周用1%阿托品)、生理盐水组。
-
46只豚鼠均以透明亚克力板鼠笼饲养于清洁级动物房内,采用12 h-12 h昼夜循环节律,光照度保持在400~500 lux。采用头套法[12]对干预组豚鼠随机选择单眼进行形觉剥夺近视造模,并定义为模型眼,对侧暴露眼为自身对照眼,所有干预组豚鼠佩戴头套4周,每日上午8:00至10:00按分组对模型眼进行滴眼给药(优化组1前2周使用1%阿托品,后2周使用0.01%阿托品滴眼;优化组2前2周使用0.01%阿托品,后2周使用1%阿托品滴眼);空白对照组豚鼠不采取任何处理。
-
在暗室中用红外偏心摄影验光仪(昆山德梅内克机器人科技有限公司)测量小鼠眼球的屈光度。测量前将豚鼠置于暗室中至少5 min以适应光线,随后将其放在距摄像头前1 m的可移动架子上,调整豚鼠位置,当被测眼的浦肯野像出现在瞳孔中央时,记录测量屈光度值。每只眼测量5次,取平均值用于分析。
-
使用实验动物A超(Strong 6000A, 武汉思创眼科)测量眼轴数值。测量前先对豚鼠进行眼球表面麻醉,后将探头垂直轻贴眼球表面,待波形图呈现完整峰型时机器自动记录数值。每眼测量8次,取均值用于分析。
-
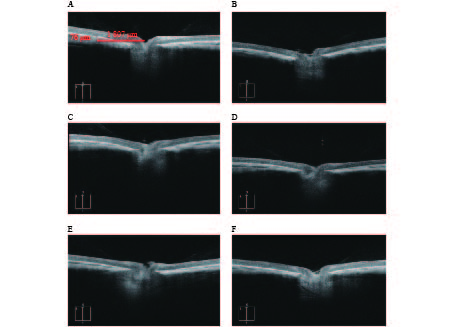
豚鼠全身麻醉后立即用Micron OCT成像系统(Phoenix)测量豚鼠脉络膜厚度。麻醉前每5 min用复方托吡卡胺滴眼液散瞳,共3次。用缝合线将豚鼠上结膜吊起,角膜表明涂抹氧氟沙星眼膏作为耦合剂和抗菌剂,将光学探头按照视轴方向抵近接触角膜中央区,屏幕上可见实时二维光学切片图,取距离视盘中心1 807 μm处测量脉络膜厚度6次,取均值。
-
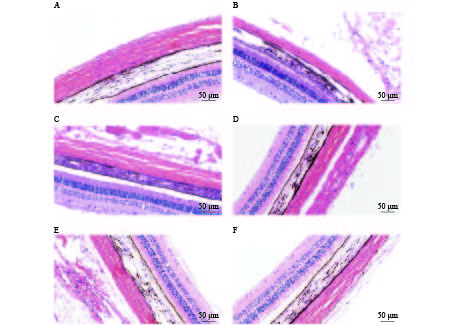
将麻醉后的豚鼠固定于操作台,打开胸腔,暴露心尖,用静脉输液针插入左心房并注入预冷的组织固定液,待心脏膨起后立即剪断右心耳,并继续缓慢推入组织固定液,待循环血全部置换后迅速解剖双侧眼球,放入眼球固定液,并做好标记。对标本进行脱水、包埋、切片处理后采用苏木素-伊红染色进行巩膜组织结构及厚度的观察。
-
本实验所有计量资料以(
$\bar x \pm {\rm{s}}$ )表示。采用软件SPSS 25.0进行数据的统计分析,P<0.05为差异有统计学意义。 -
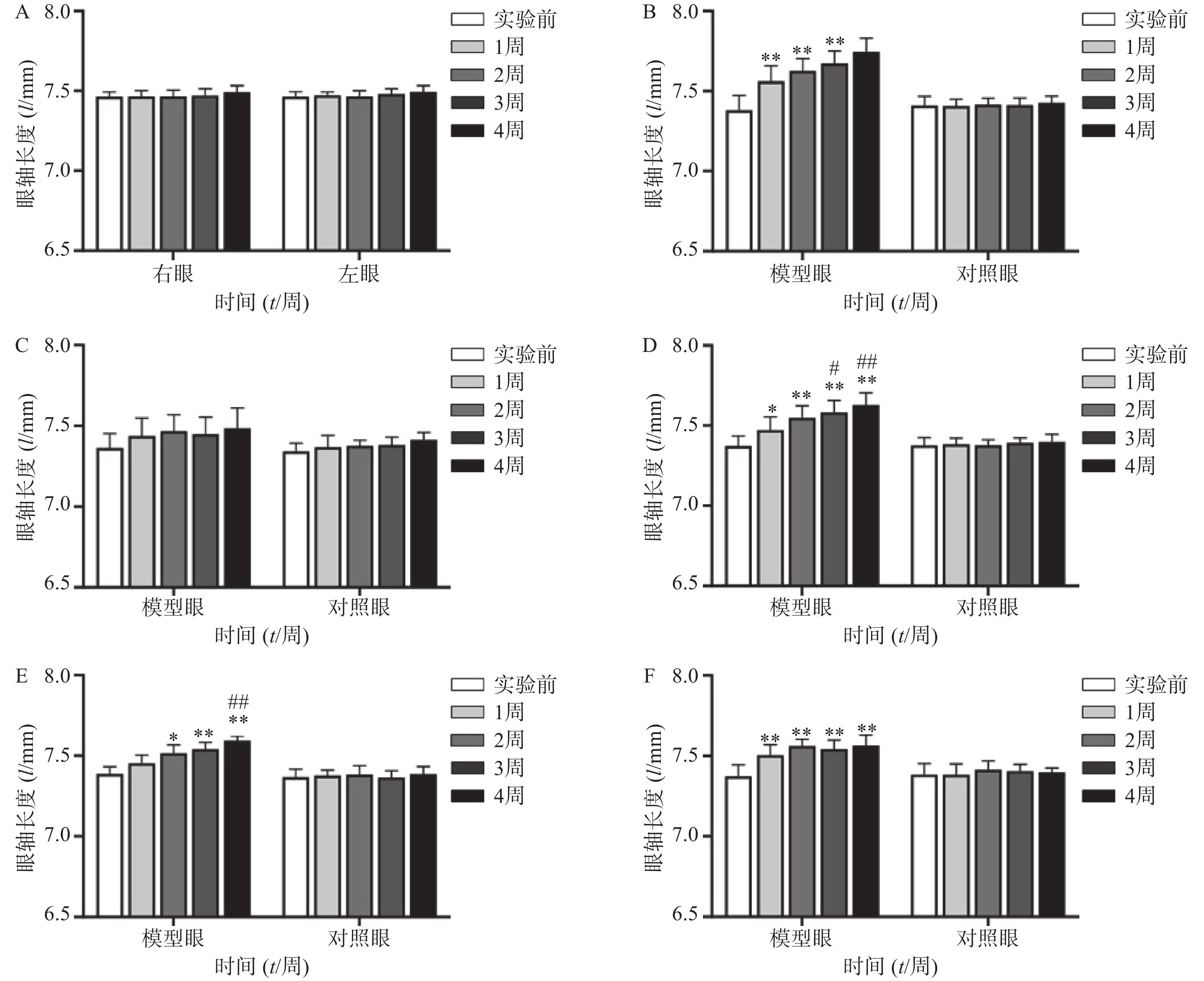
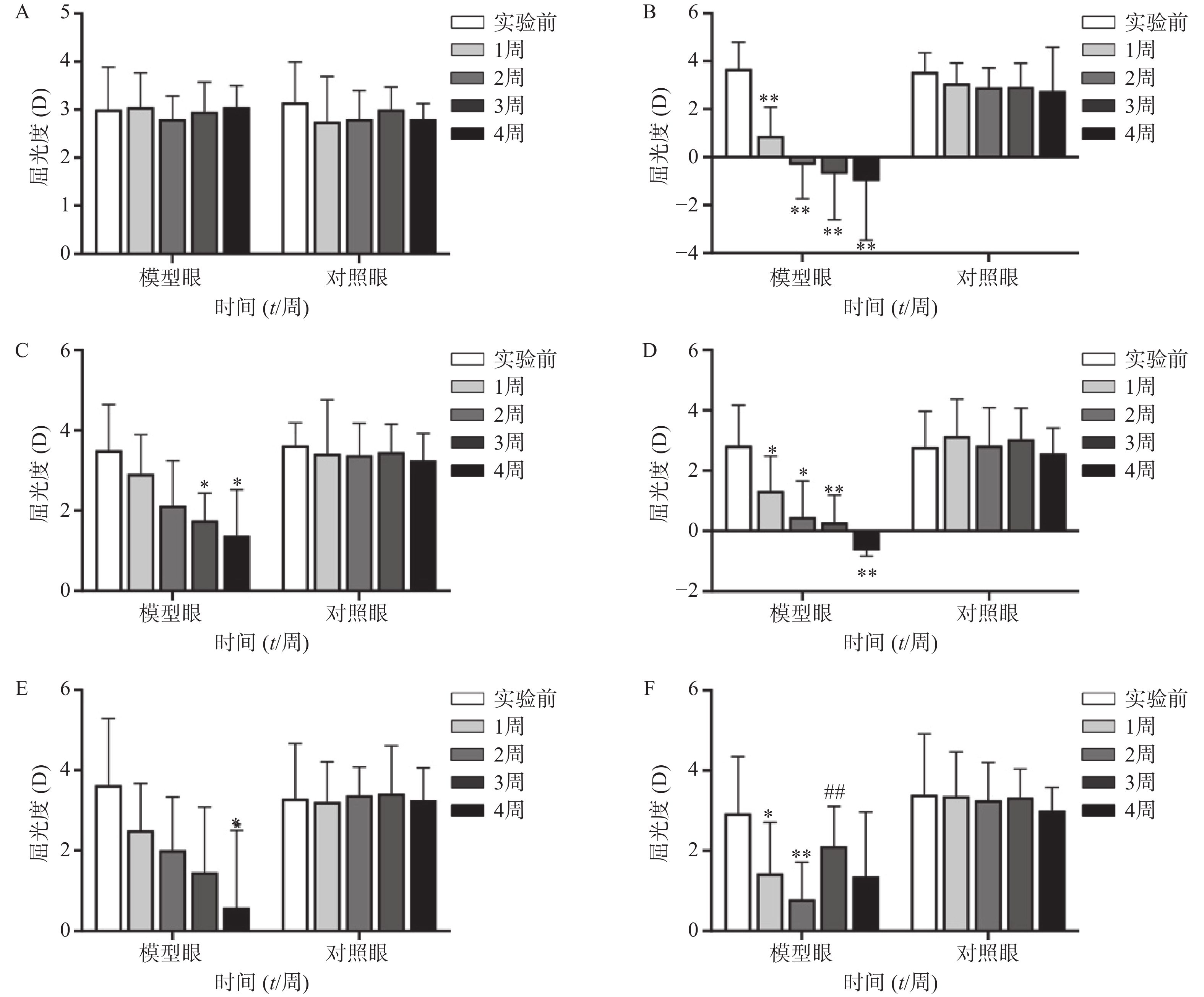
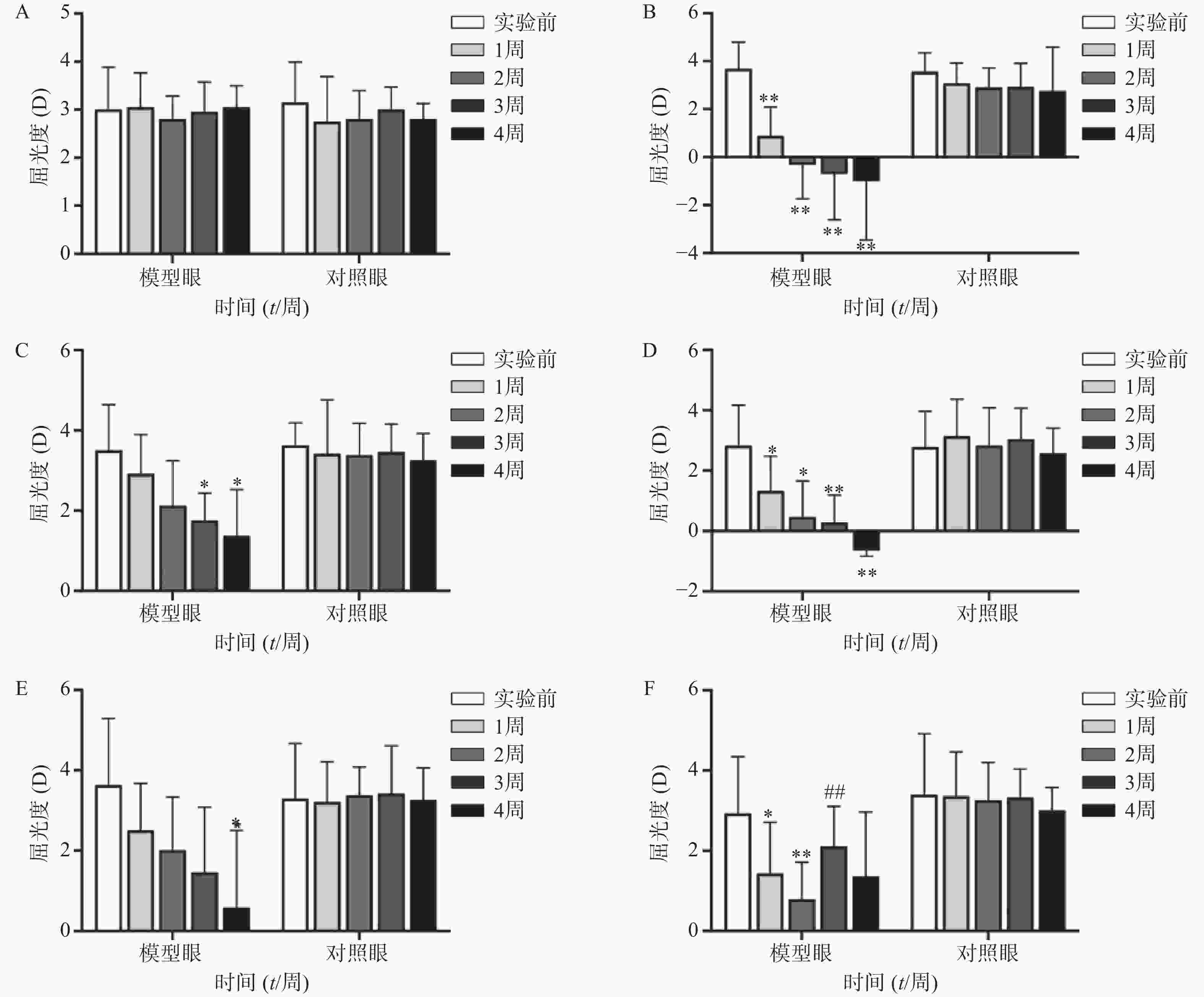
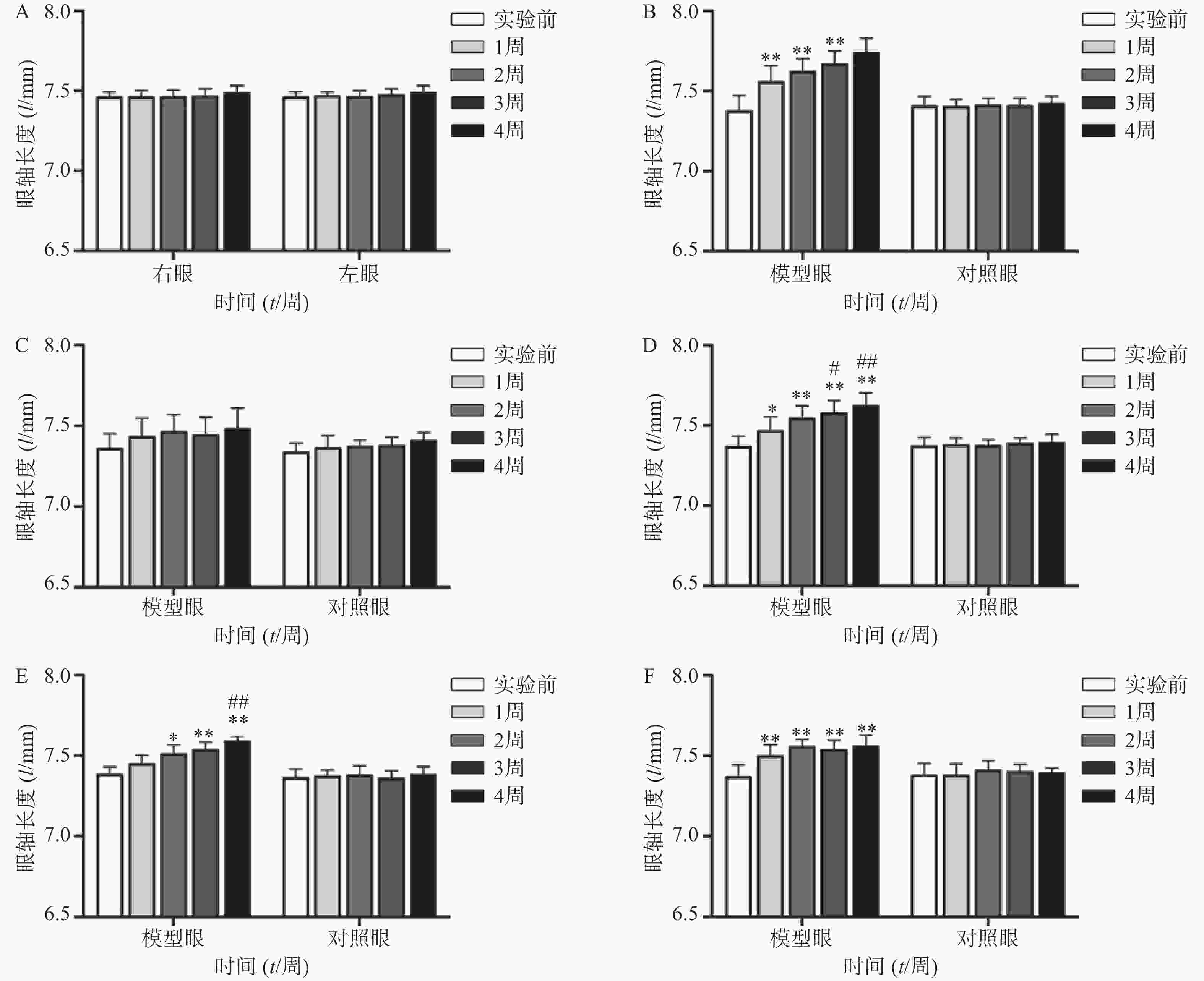
空白对照组(图1A、2A)实验前后双眼屈光度和眼轴差异均无统计学意义(P>0.05)。生理盐水组(图1 B)屈光度和眼轴长度在1周末时为(0.88±1.21)D和(7.56±0.10)mm,与实验前(3.68±1.12)D和(7.38±0.09)mm相比有显著性差异(P<0.01),2周末时该组出现绝对近视(−0.33±1.41)D和(7.62±0.08 mm),4周末时屈光度和眼轴持续呈近视化改变(−1±2.47)D和(7.74±0.09)mm。给药各组模型眼屈光度、眼轴长度均有不同程度近视化改变。各干预组对照眼的屈光度、眼轴长度于实验前后均无显著差异(P>0.05)。以上结果说明豚鼠近视模型建立取得成功。
-
空白对照组(图1A)双眼屈光度于实验前后均无统计学差异(P>0.05);生理盐水组(图1B)模型眼屈光度迅速下降,每周测量数值均与实验前有显著差异(P<0.01);1%阿托品组(图1C)模型眼屈光度呈下降趋势,于第3、第4周末时分别降至(1.75±0.69)D和(1.38±1.15)D,均与实验前(3.50±1.14)D有显著差异(P<0.05);0.01%阿托品组(图1D)模型眼屈光度快速下降,在第1周末时与实验前相比即存在显著性差异[(1.32±1.15)D 对(2.82±1.35)D, P<0.01],4周末时出现绝对近视(−0.64±0.20)D;优化组1(图1E)模型眼屈光度缓慢下降,第4周末时的屈光度值为(0.95±1.90)D,与实验前[(3.55±1.85)D]相比有统计学差异(P<0.01),该组抑制屈光度下降结果低于1%阿托品组,但高于0.01%阿托品组。优化组2(图1F)模型眼实验前2周快速下降,第2周末时的屈光度(0.79±0.93)D与实验前(2.93±1.42)D相比有显著性差异(P<0.01);该组第3周换用1%阿托品后,屈光度回升至(2.10±1.00)D,与第2周末的屈光度值有显著性差异(P<0.01);第4周末时屈光度值下降至(1.36±1.61)D,与实验前无统计学差异(P>0.05)。各组对照眼实验前后屈光度无统计学差异(P>0.05)。
-
空白对照组(图2A)双眼眼轴长度于实验前后均无统计学差异(P>0.05);生理盐水组(图2B)模型眼眼轴迅速延长,每周测量数值均与实验前有显著差异(P<0.01);1%阿托品组(图2C)模型眼眼轴长度于实验前后均无统计学差异(P>0.05);0.01%阿托品组(图2D)模型眼于实验1周时出现了明显的眼轴延长(7.47±0.08)mm对(7.37±0.06)mm,于第4周末时该组眼轴延长至(7.62±0.08)mm,均与实验前有显著差异(P<0.05);优化组1(图2E)模型眼造模前眼轴长度为(7.39±0.05)mm,于第2周末时眼轴长度明显延长(7.52±0.05)mm,P<0.05,第3、4周使用0.01%阿托品治疗后,2周内眼轴延长速度有所减缓[(7.54±0.04)mm和(7.59±0.03)mm];优化组2(图2F)模型眼眼轴在实验前2周迅速延长,造模前眼轴长度为(7.37±0.07)mm,实验1周时出现了明显的眼轴延长[(7.50±0.07)mm, P<0.01],第3、4周使用1%阿托品治疗后眼轴长度为(7.54±0.06)mm和(7.56±0.05)mm,与第2周末相比差异无统计学意义(P>0.05)。各组对照眼实验前后眼轴长度无统计学差异(P>0.05)。
-
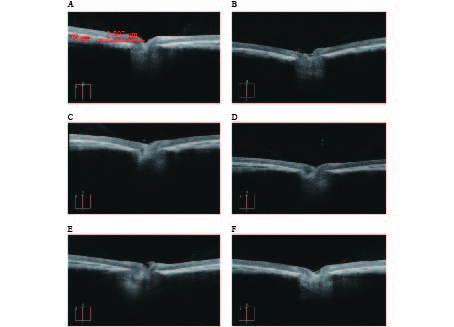
OCTA结果提示,空白对照组脉络膜结构完整,边界清晰。干预组模型眼脉络膜均出现不同程度的结构和形态的改变,如组织厚度下降,边界模糊等(图3)。OCTA结果可见各干预组脉络膜厚度差别较为明显,大小依次为:1%阿托品组>优化组2>优化组1> 0.01%阿托品组>生理盐水组(表1)。
组别 脉络膜厚度(l/μm) 空白对照组 72.25±3.30**## 生理盐水组 33.60±2.19 1%阿托品组 57.00±1.73 0.01%阿托品组 41.00±4.08**## 优化组1 48.67±0.58**## 优化组2 51.33±2.52**# * P<0.05、** P<0.01,与空白对照组比较; # P<0.05、## P<0.01,与1%阿托品组比较 -
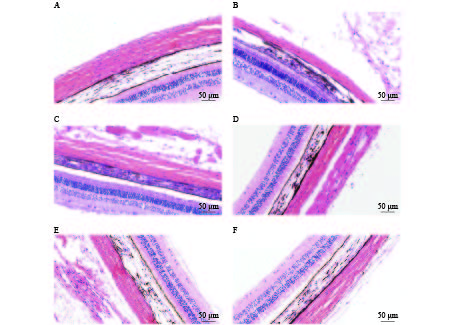
HE染色结果提示,空白对照组巩膜组织胶原纤维排列整齐、分布均匀、连接致密。各干预组模型眼的巩膜存在不同程度的组织结构改变,如组织厚度变薄、胶原纤维粗细不均、排列稍紊乱、组织间隙变大等(图4)。HE染色可见各组巩膜厚度的差别较明显,空白对照组巩膜厚度为(91.03±1.68)μm,各干预组豚鼠模型眼的巩膜厚度均小于空白对照组,厚度大小依次为:1%阿托品组>优化组2 >优化组1> 0.01%阿托品组>生理盐水组(表2)。
组别 巩膜厚度(l/μm) 空白对照组 91.03±1.68**## 生理盐水组 39.17±2.15 1%阿托品组 74.4±1.95** 0.01%阿托品组 54±2.99**## 优化组1 63.43±2.10**## 优化组2 67.40±2.79**# * P<0.05,** P<0.01,与空白对照组比较; # P<0.05,## P<0.01,与1%阿托品组比较。 -
阿托品是非选择性M受体拮抗剂,从19世纪就开始用于近视治疗,目前仍在临床广泛使用。大量研究证实阿托品是目前控制近视进展最有效的药物[13-14],也是临床上治疗近视使用度最高的药物,但是在临床应用过程中1%阿托品的副作用一直备受争议。国内外已有大量研究报道0.01%阿托品在长期控制近视中有较好效果,并且其副作用及停药反弹现象较轻,但是短期内对于迅速发展的近视控制效果欠佳[6, 11]。本研究结合不同浓度阿托品的作用优势和临床实际提出优化方案1,动物实验证明优化方案1同样能有效控制屈光度的快速发展,但是该方案对于抑制眼轴增长作用和对脉络膜、巩膜形态保护作用不如1%阿托品组,可能因为0.01%浓度过低,对组织、形态上作用力不足。此外,有研究表明,给予明亮光照和阿托品可协同刺激眼内多巴胺的生成,有助于延缓近视发展[15],但是1%浓度的阿托品因畏光发生率为62.12%(205/330,中国香港)[16],对于联合光照治疗有较大局限,而0.01%阿托品的畏光发生率为2.1%(2/110,中国云南)[17],1%阿托品有效控制近视发展后,采取0.01%阿托品结合沐浴阳光疗法维持防治效果也为近视治疗提供一种可能。近期有学者针对0.01%~0.1%浓度进行临床随机对照研究,发现较低浓度的阿托品滴眼液如0.05%、0.025%表现出更好的防控效果,在2年内最有效地控制了屈光度进展和眼轴延长,且对视力相关生活质量均无不良影响 [17-19],提示在0.01%和0.1%浓度阿托品之间还存在更优治疗浓度,因此本研究的优化方案1中维持治疗浓度还需进一步探究。
脉络膜和巩膜在形觉剥夺性近视和离焦性近视中对于眼焦调整以及眼球生长调节中发挥重要作用。动物研究发现,近视眼中均存在不可逆脉络膜、巩膜变薄的情况,临床同样证明,脉络膜、巩膜厚度与近视的严重程度高度相关[19-20]。近年来,国外临床上常使用0.01%阿托品治疗早期近视,或对远视储备不足的患者进行预防治疗[20]。但是其在短时间内对于快速发展的近视控制效果欠佳,对于脉络膜和巩膜的保护作用效果不如1%阿托品[21]。优化方案2发现,该组给药方案对于脉络膜和巩膜纤维层结构和形态的维持作用接近1%阿托品组,并且明显优于0.01%阿托品组。值得注意的是,当0.01%阿托品换为1%阿托品后,近视发展方向被翻转,明显向远视方向移动,4周末时该组对屈光度控制效果优于1%阿托品组,并且眼轴增长速度较0.01%阿托品组明显缓慢。该结果提示,在近视早期治疗时使用0.01%阿托品,当近视控制效果不佳时换用高浓度阿托品(如1%)对于近视治疗时机并未产生影响,也未加重脉络膜和巩膜结构的不可逆改变。这一结果与Wu等[22]在临床实验中得到的结论一致,即从低浓度阿托品(如0.01%)开始治疗,当控制效果不够有效(≥0.5 D/6个月,亚洲)时,可以考虑增加阿托品浓度代替治疗。
本研究也存在一定局限性。与临床研究周期相比,本研究观察时间尚短,不能提供长期使用的控制效果数据。并且,在实验期间报告了几种给药方案的治疗效果,但未进行停药后效果的监测,该部分在下一步的研究中会开展相应的实验设计。此外,优化组2换用1%阿托品后,模型眼屈光度出现翻转的现象。本研究尚未对此深入探究,目前无法判断具体影响因素,对于翻转作用的机制未来需要作进一步研究。
Study on the effect of optimized dosing regimen of atropine on the treatment of myopia in guinea pigs
doi: 10.12206/j.issn.1006-0111.202104119
- Received Date: 2021-04-27
- Rev Recd Date: 2021-07-23
- Available Online: 2021-09-28
- Publish Date: 2021-09-25
-
Key words:
- myopia /
- atropine /
- form deprivation /
- guinea pigs
Abstract:
| Citation: | CHI Qingqing, ZHANG Xue, MU Wan, YIN Yao, CHEN Meng. Study on the effect of optimized dosing regimen of atropine on the treatment of myopia in guinea pigs[J]. Journal of Pharmaceutical Practice and Service, 2021, 39(5): 409-414, 464. doi: 10.12206/j.issn.1006-0111.202104119 |








 DownLoad:
DownLoad: