-
外周神经系统(PNS)是中枢神经系统发出,导向人体各部分的神经,也称周围神经系统。外周神经的主要成分是神经纤维,包括传入神经纤维和传出神经纤维。外周神经损伤约占所有创伤的2.8%[1],可导致患者的感觉与运动功能发生障碍。外周神经损伤后机体自身有一定的再生能力但修复速度很慢,因此,借助药物途径促进外周神经再生修复是临床治疗的关键[2-3]。了解与外周神经再生相关的信号通路,有助于外周神经损伤后修复药物的研发。
HTML
-
雪旺细胞(Schwann cells,SCs)是PNS的支持细胞,可以分化为PNS的髓鞘,它能增殖,迁移到受损神经区域的远端,对于维持外周神经损伤后再生有着重要作用。外周神经损伤后,外周神经系统中的成熟神经元也可以通过增强内在的生长能力来再生轴突。以下主要介绍这两类细胞中与外周神经再生相关的信号通路。
-
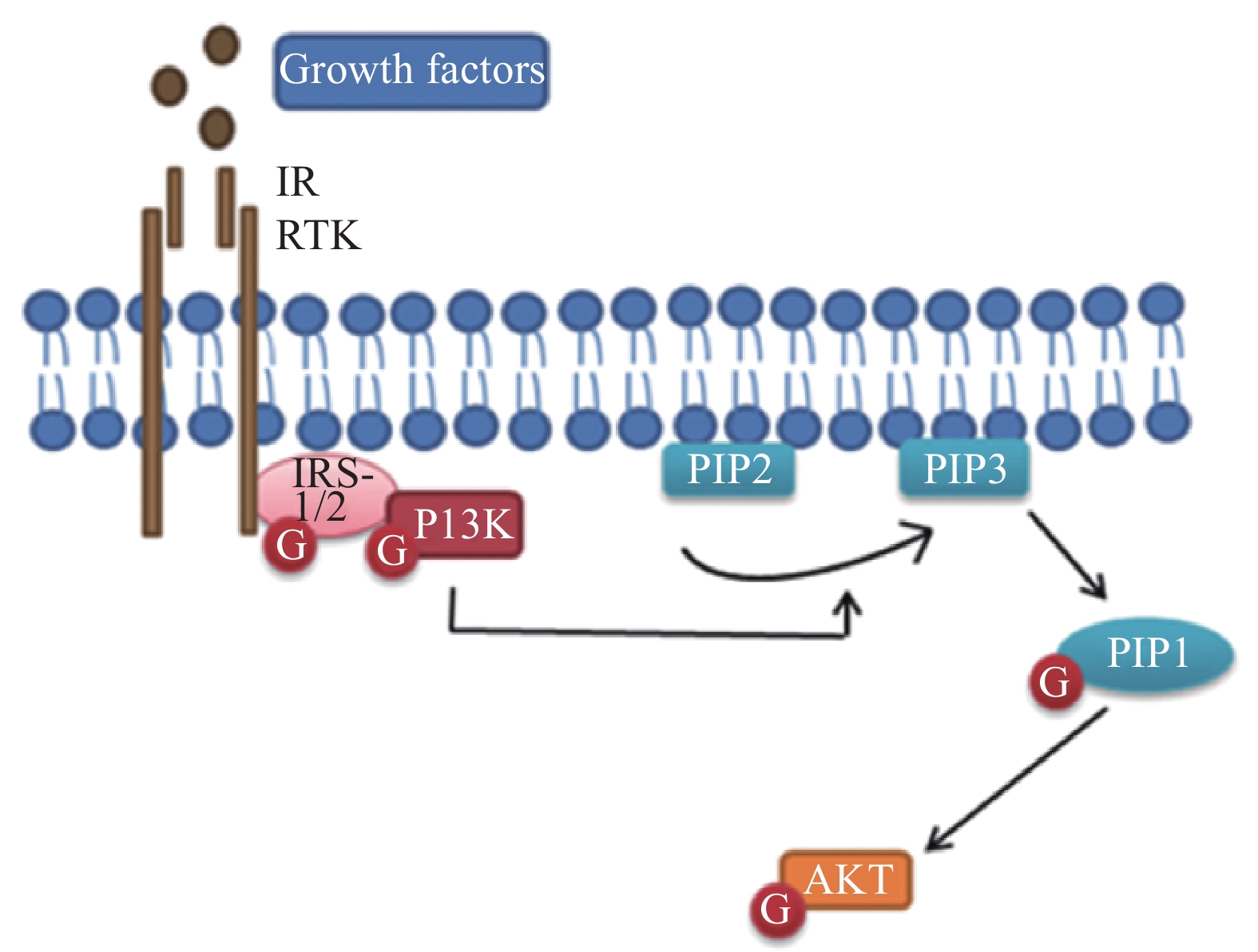
蛋白激酶B(Akt)被认为是髓鞘形成过程中磷酸肌醇3激酶(PI3K)的主要效应物,Akt的激活增加了髓磷脂蛋白的表达,包括与髓鞘再生相关的髓磷脂碱性蛋白(MBP)和髓鞘蛋白(MPZ)的表达。有研究证明,用LY294002(PI3K活性抑制剂)抑制PI3K/Akt途径可显著减弱SCs的迁移[4-5]。此外,该信号通路的抑制也明显降低了SCs中增殖细胞核抗原(PCNA)的合成[6]。这些发现表明,PI3K/Akt途径参与了外周神经损伤后的修复过程,相关的信号通路见图1。
-
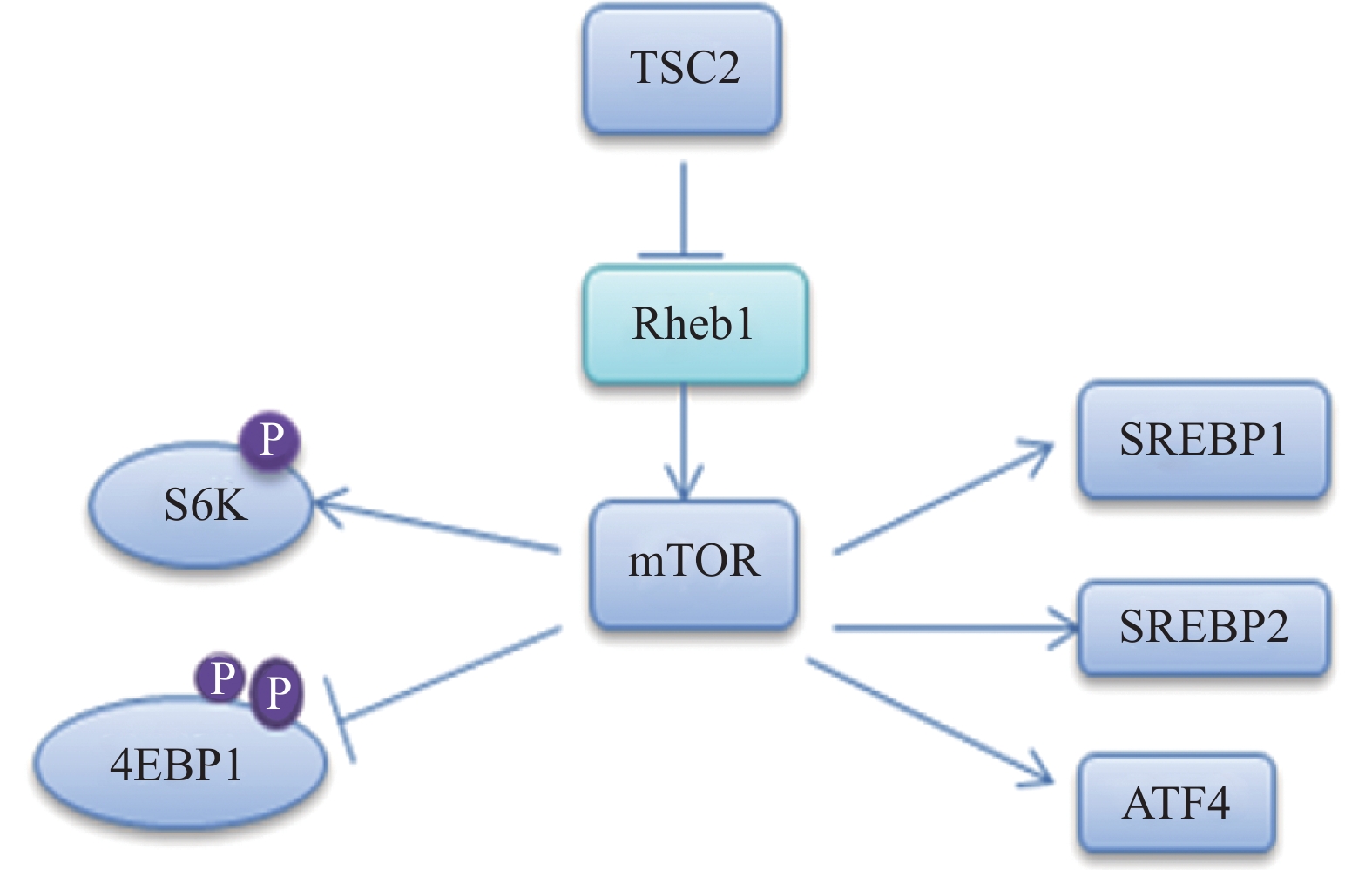
髓磷脂的形成需要合成大量的脂质,尤其是胆固醇。雷帕霉素靶蛋白(mTOR)包含两个核蛋白(mTORC1和mTORC2)。在SCs中,mTORC1通过甾醇调节元件结合转录因子(SREBP)1和2调节脂质的合成[7-8],其中,SREBP1调节脂肪酸和甘油三酰的合成,SREBP2主要是调节胆固醇合成[9],相关的信号通路见图2。
-
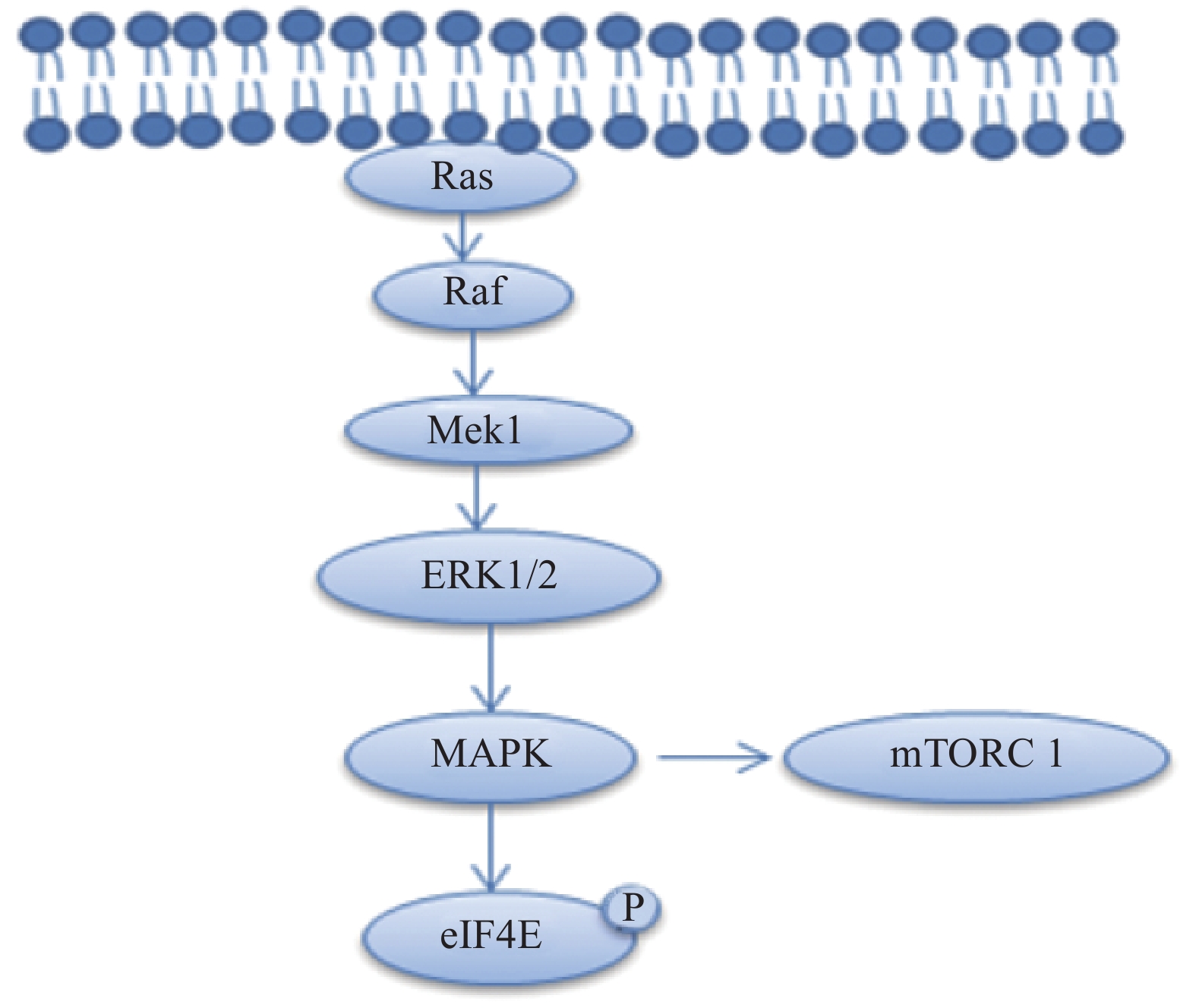
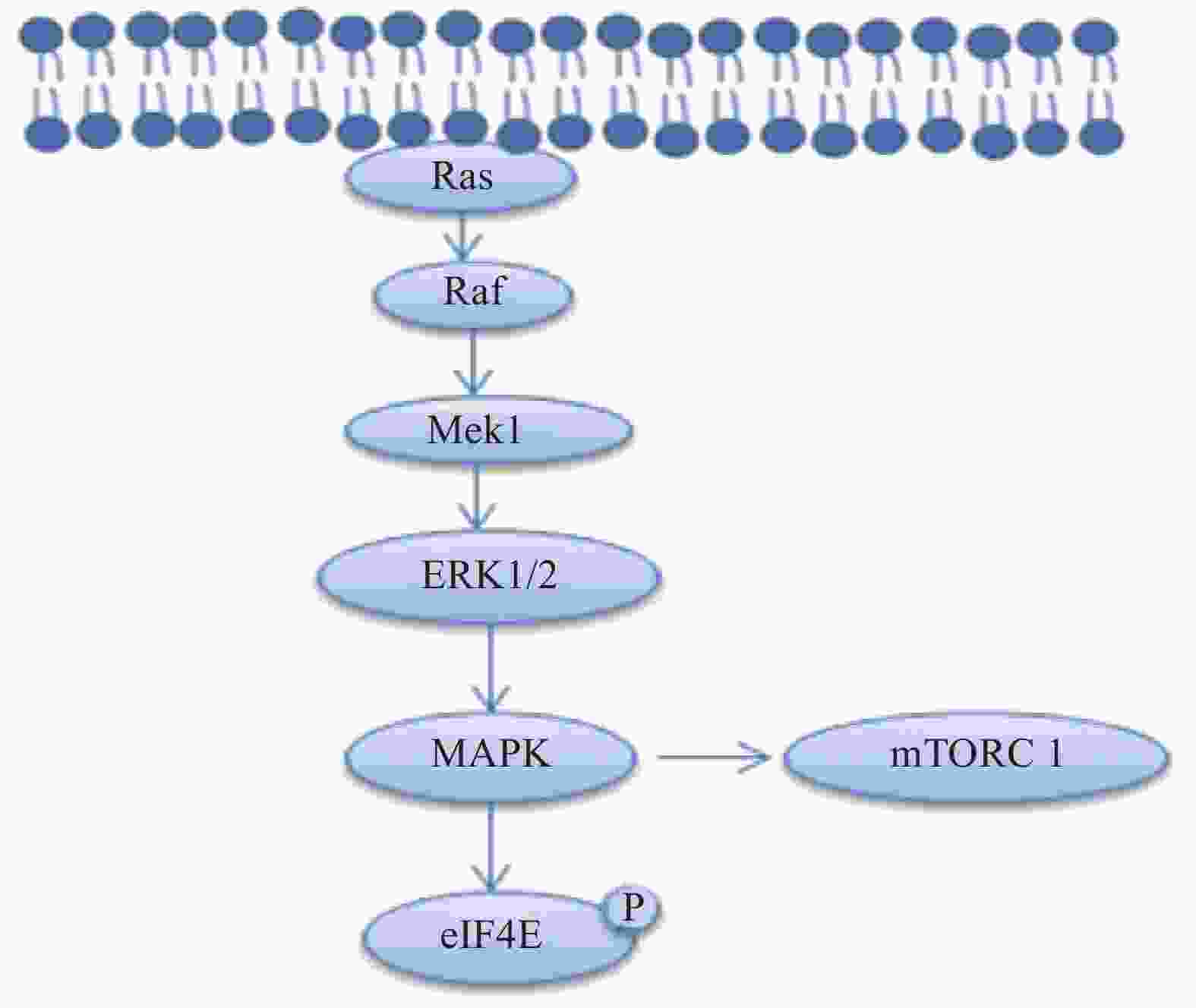
丝裂原活化蛋白激酶激酶1(MAP kinase kinase1, Mek1)通过磷酸化细胞外信号调节蛋白激酶(extracellular signal-regulated kinases,ERK1/2)激活丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)的信号传导,MAPK信号传导通过磷酸化eIF4E,S6K和4EBP1蛋白,增强雪旺细胞中的髓磷脂蛋白和控制脂质生物合成的酶的合成[10-11],相关的信号通路见图3。
-
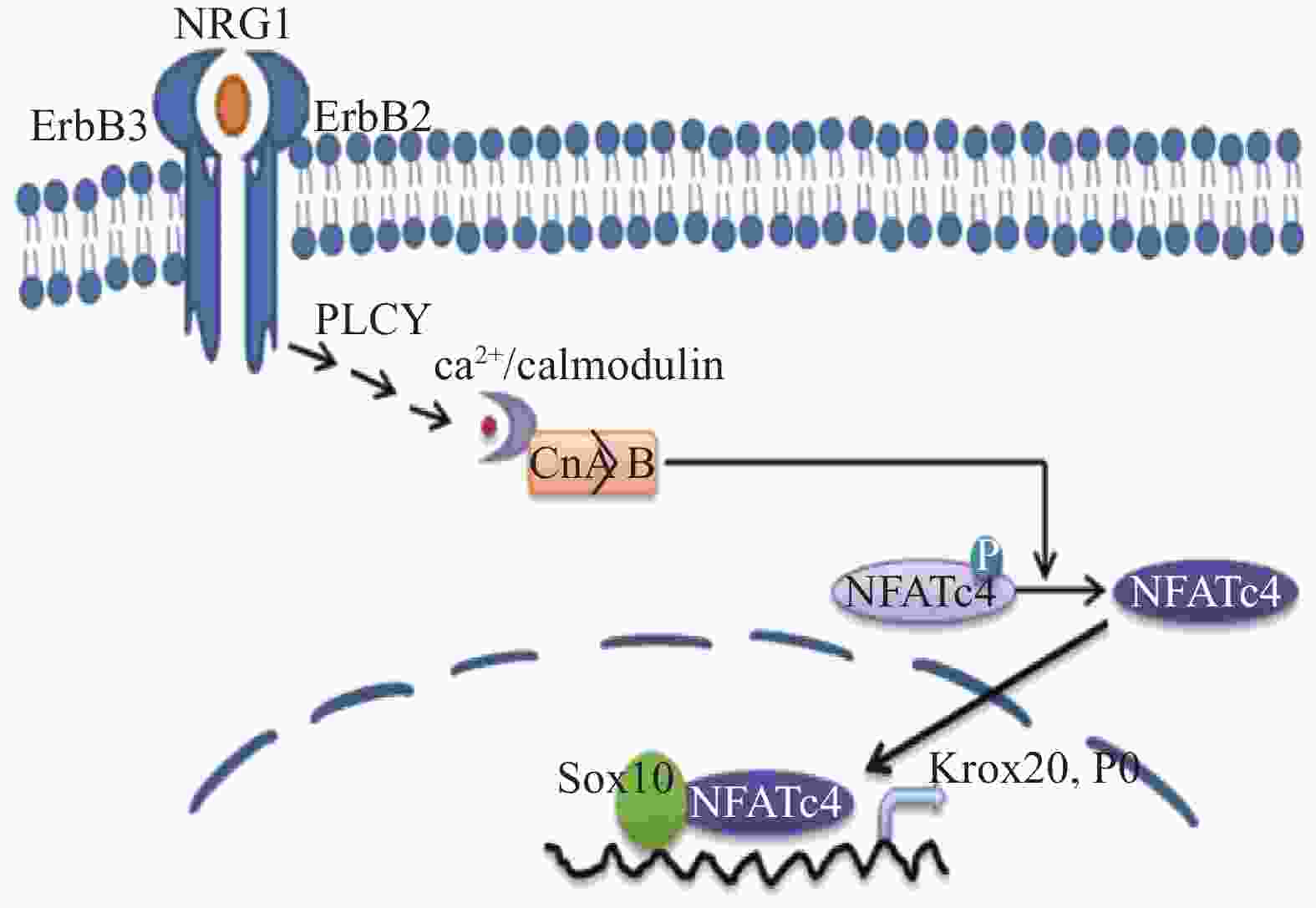
神经调节蛋白1(NRG1)刺激Ca2+内流,细胞内Ca2+水平的持续增加激活细胞质中的钙调神经磷酸酶,使活化的T细胞核因子(nuclear factor of activated T-cells,NFATc)蛋白去磷酸化,进入细胞核并组装成 NFAT 转录复合物。钙调神经磷酸酶/NFAT信号传导通过两个途径调节雪旺细胞的发育:一是通过作用于神经调节蛋白和酪氨酸激酶受体(ErbB)的下游,在髓鞘前期形成阶段直接激活转录激活因子(Krox20),进而促进髓鞘基因的表达。另一种是通过在髓鞘形成阶段激活编码髓鞘结构蛋白的基因,如 P0[12],相关的信号通路见图4。
-
NRG1是神经元和神经胶质细胞存活、增殖、分化和迁移所必需的一系列生长因子之一[13-14],其中NRG1的β形式(NRGβ1)可以通过加速外周神经损伤后的SCs迁移来促进神经再生。NRGβ1先是与ErbB2/3受体结合,随后促进黏着斑激酶(focal adhesion kinase, FAK)磷酸化并激活与迁移活性相关的下游信号的转导[15-16],SCs的迁移对于外周神经损伤后的再生至关重要。
-
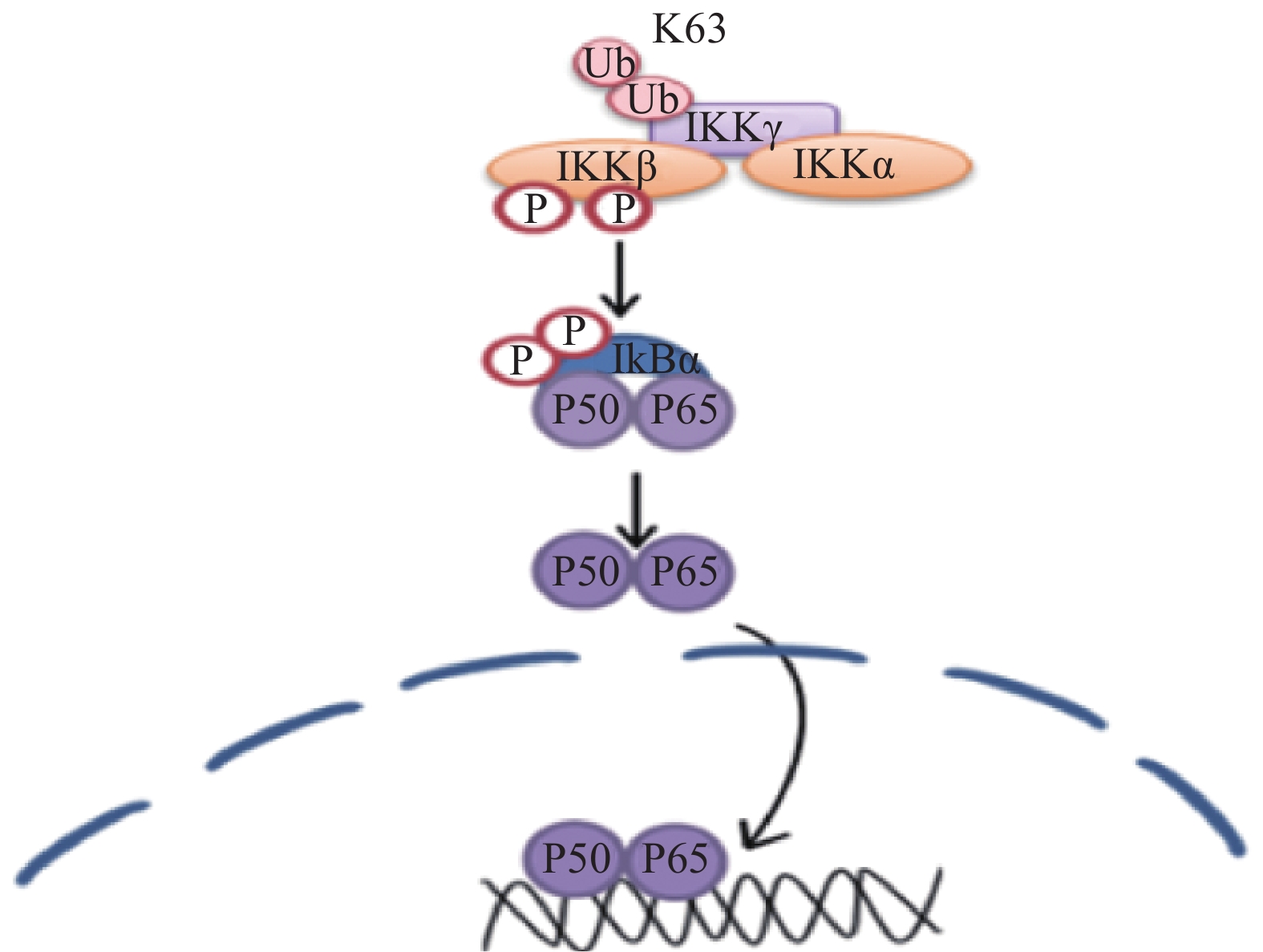
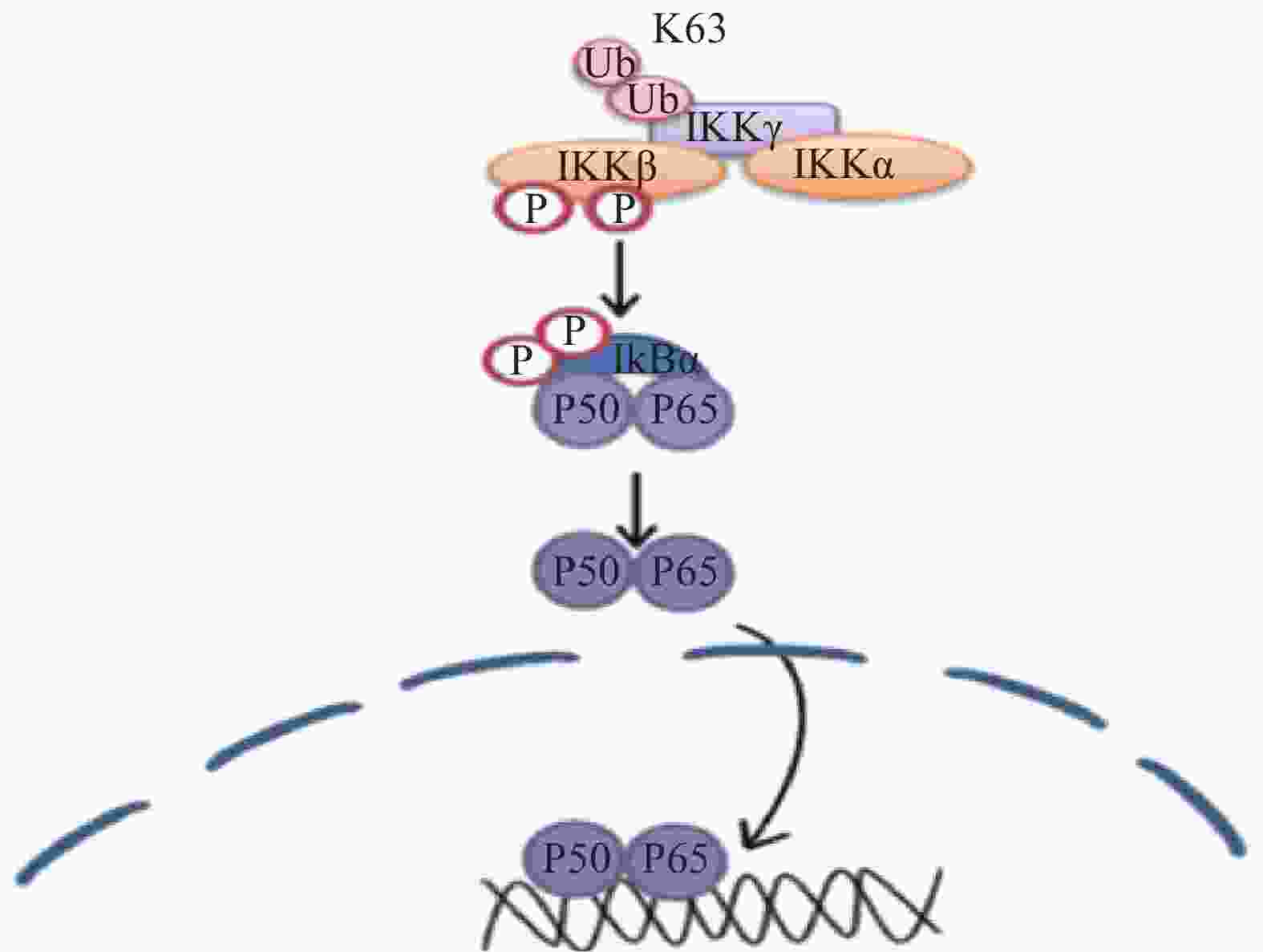
核转录因子(NF-κB)可能在正常生理条件下正调控Krox-20的表达,Krox20 是对PNS髓鞘形成至关重要的转录因子,在外周神经髓鞘形成过程中,Krox-20部分地调节胆固醇/脂质生物合成基因的表达。有研究表明,Krox-20和SREBP的反式激活剂可协同激活许多SREBP靶基因的启动子,从而调节髓鞘的形成[17],相关的信号通路见图5。
-
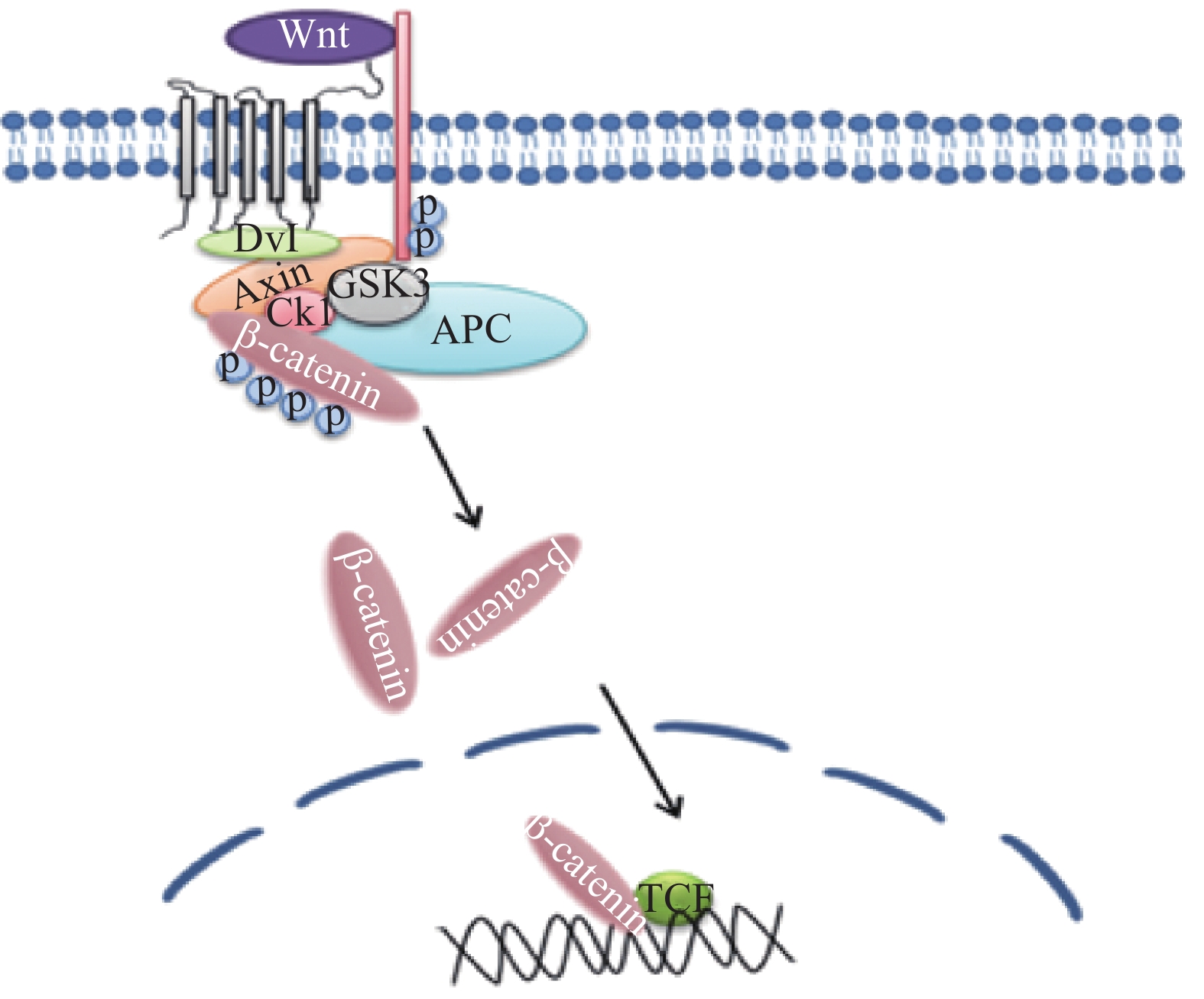
Wnt/β-catenin途径参与雪旺细胞髓磷脂基因启动子活性的调控,促进雪旺细胞中髓磷脂蛋白基因的表达。在跨膜受体(frizzled, FZD)蛋白家族接收Wnt信号后,可通过下游蛋白激酶的磷酸化作用抑制β-catenin的降解活性,随后细胞质中积累高水平的β-catenin蛋白易位进入细胞核,与转录因子(T cell factor/lymphoid enhancer factor, TCF/LEF)形成复合物,并激活与细胞存活、增殖和分化相关的目标基因[18]。有研究发现抑制Wnt/β-catenin信号传导途径,将抑制雪旺细胞的增殖[19],相关的信号通路见图6。
-
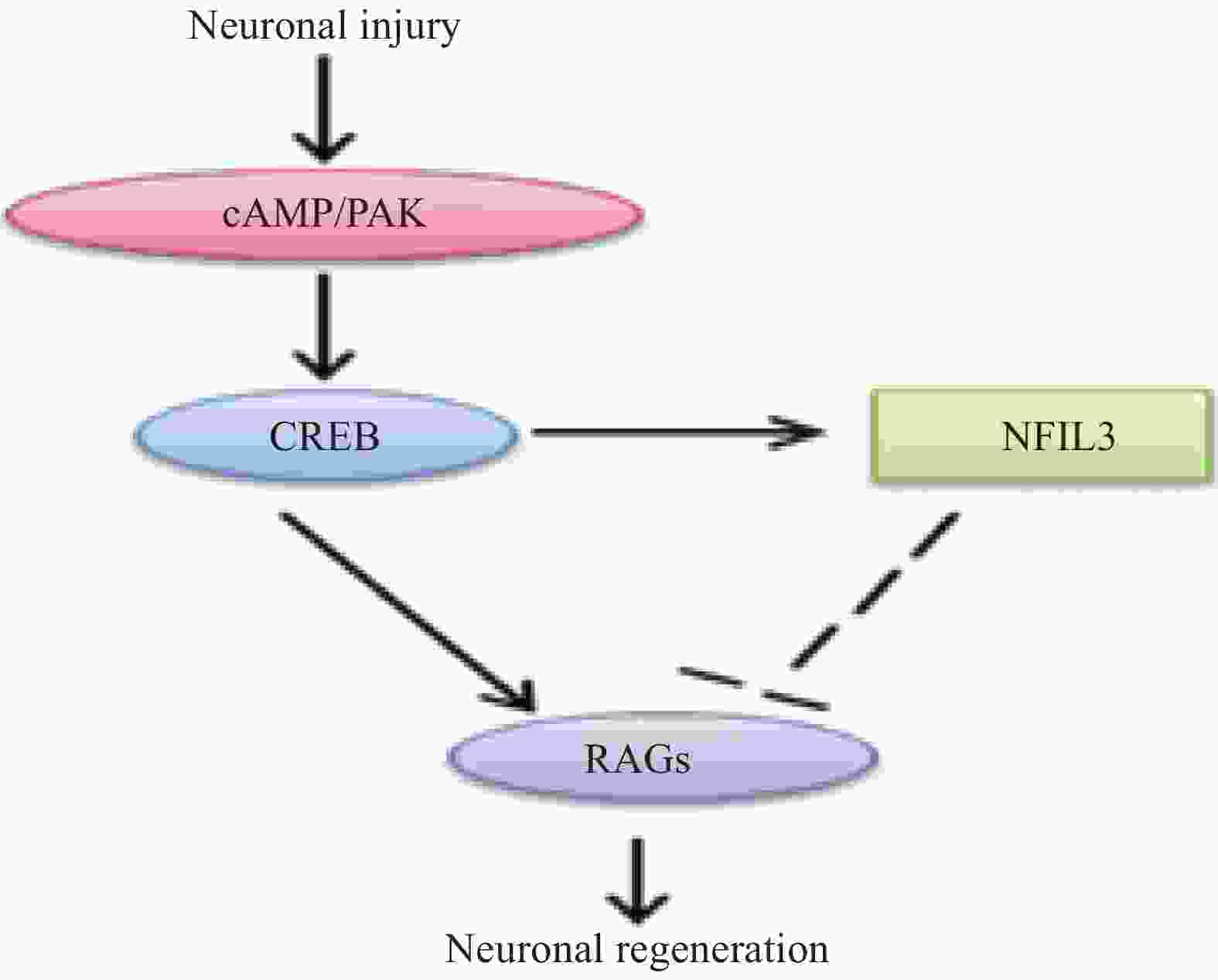
外周损伤后,钙依赖性腺苷酸环化酶产生的cAMP影响cAMP反应元件结合蛋白1 (CREB1)的活性,并调节靶基因的表达,进而影响神经元的生长。另外,在外周神经损伤后,cAMP 信号通路也能促进PAK等促再生信号通路的激活[20],相关的信号通路见图7。
-
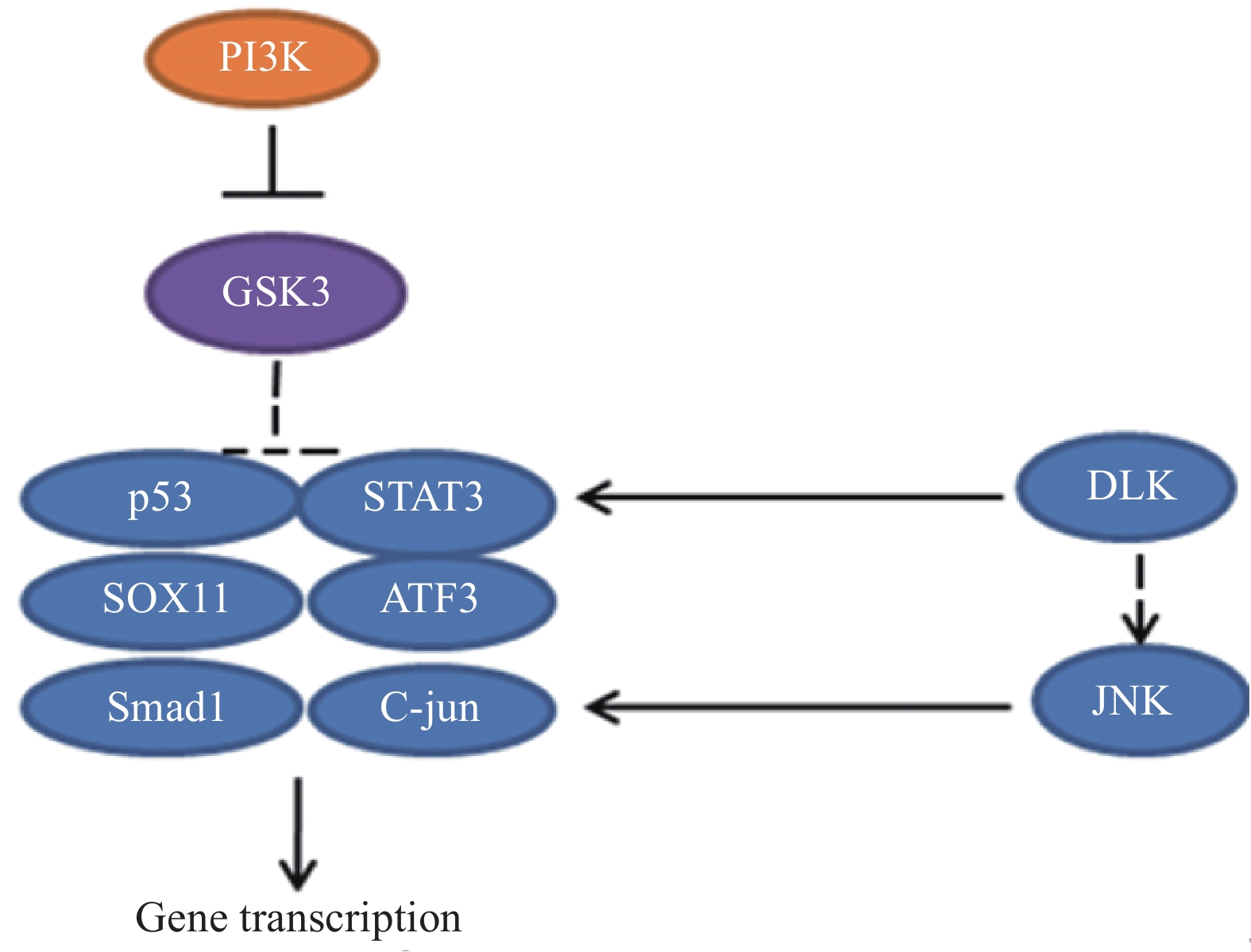
在神经元损伤后,DLK诱导下游应激信号,包括磷酸化的c-Jun氨基末端激酶(c-Jun N-terminal kinase,JNK)逆行向核传播,导致c-Jun磷酸化[21],进而促进损伤后的轴突再生[22]。最近的研究揭示了DLK/JNK途径激活的机制[23],但仍未确定直接影响DLK上游的因素,相关的信号通路见图8。
-
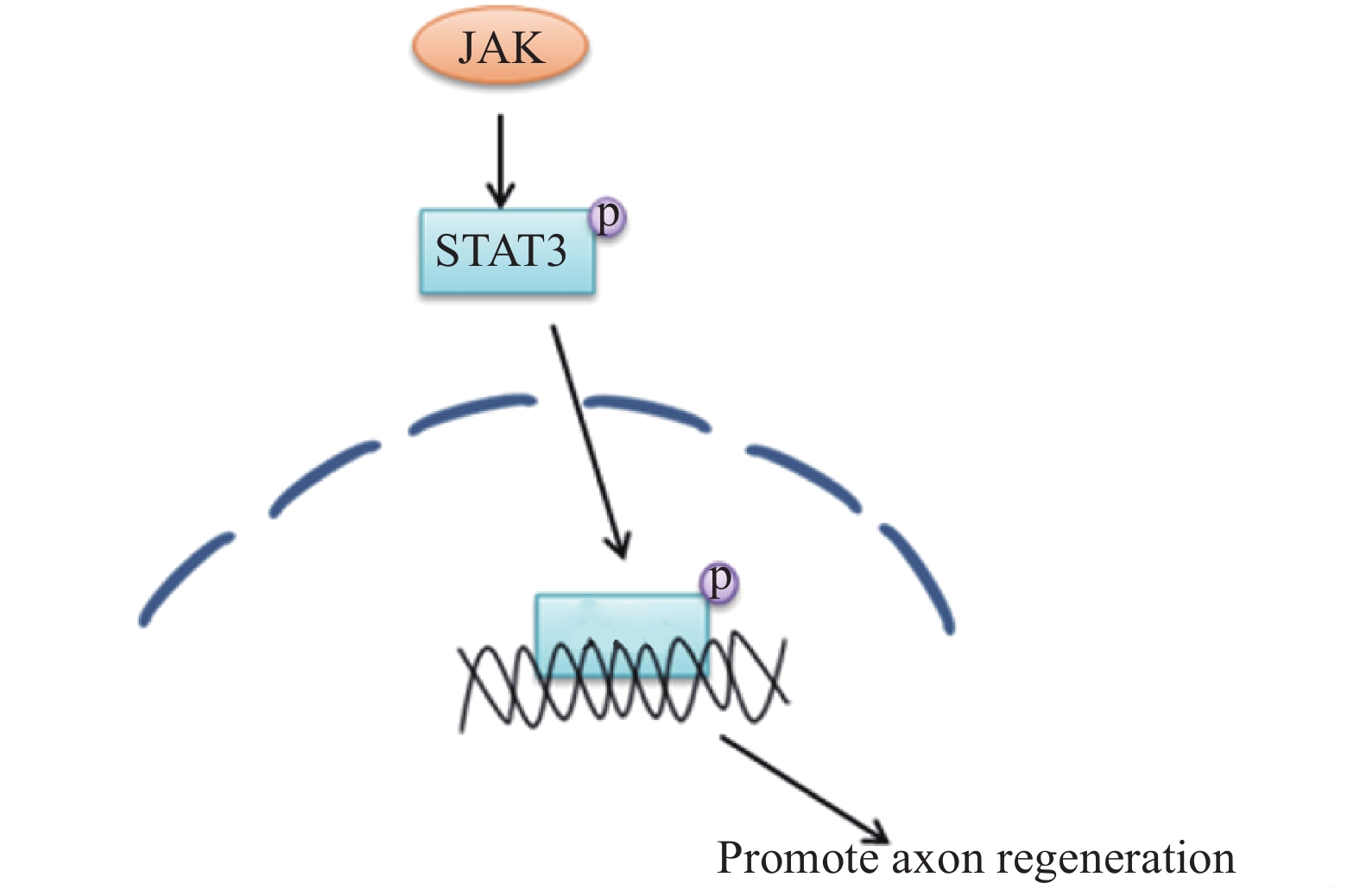
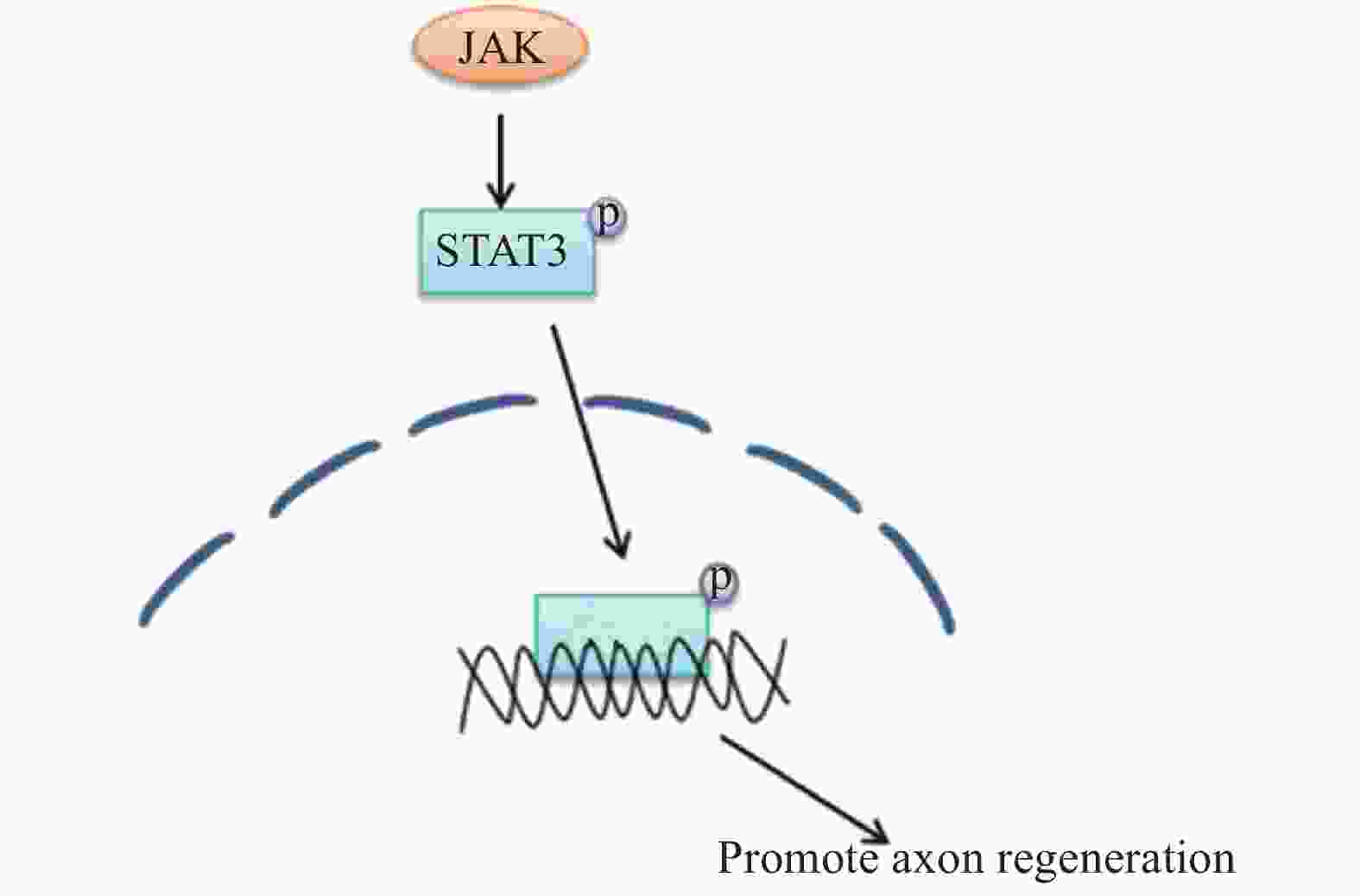
在外周神经损伤后,信号转导与转录激活因子3(STAT3)被酪氨酸激酶(JAK)磷酸化[24],磷酸化的STAT3逆行转移到细胞核中以激活再生程序,进而调节神经元的生长。因此,JAK/STAT3信号通路在增强外周神经损伤后神经元的生长能力方面有着很重要的作用[25-26],相关的信号通路见图8。
-
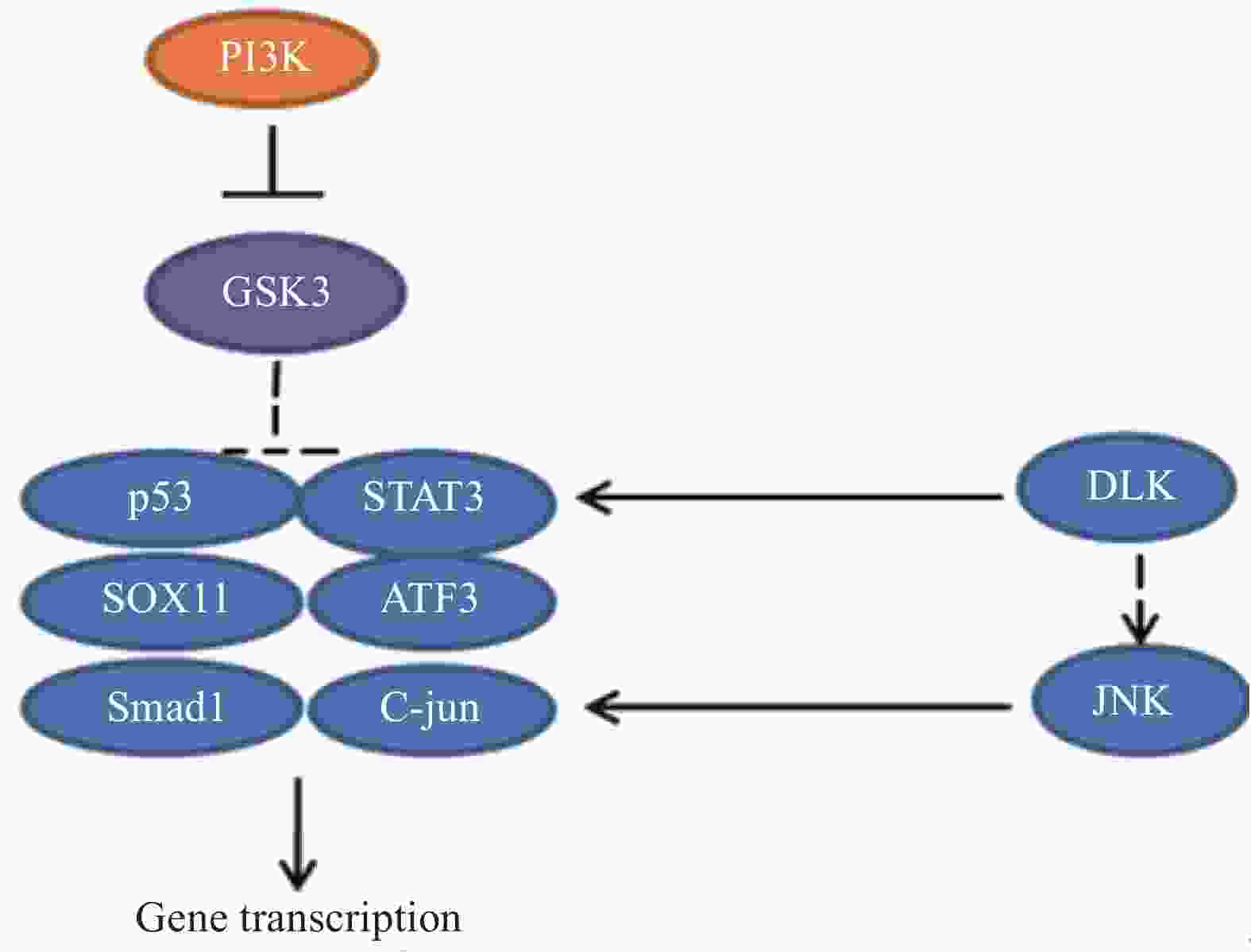
外周神经损伤激活了PI3K信号途径,导致糖原合酶激酶3(GSK3)失活,通过控制 GSK3 在胞体中的活性可以增强轴突生长能力。研究发现GSK3 是轴突再生的主要调节因子,可以在胞体和远端轴突中协调再生反应。一方面,通过抑制细胞体中的 GSK3 刺激转录依赖性轴突再生程序,增强了轴突内在生长能力。另一方面,一定程度上抑制生长锥中GSK3 活性,可以促进局部细胞骨架组装。然而,失活的程度应该被控制,较大程度抑制GSK3活性,会急剧阻断轴突生长[27-28]。相关的信号通路见图9。
1.1. 雪旺细胞中与外周神经损伤后再生的相关信号通路
1.1.1. 磷酸肌醇3激酶-蛋白激酶B (PI3K-Akt)
1.1.2. 雷帕霉素靶蛋白
1.1.3. 丝裂原活化蛋白激酶
1.1.4. 钙调神经磷酸酶/活化的T细胞核因子
1.1.5. 神经调节蛋白1-酪氨酸激酶受体-局部黏着斑激酶
1.1.6. 核转录因子
1.1.7. Wnt/β-catenin
1.2. 神经元中与周围神经损伤后再生相关的信号通路
1.2.1. 环磷酸腺苷(cAMP)
1.2.2. 双亮氨酸拉链激酶/氨基末端激酶(DLK/JNK)
1.2.3. 酪氨酸激酶/转录激活因子3
1.2.4. 磷酸肌醇3激酶-糖原合酶激酶3(PI3K-GSK3)
-
外周神经损伤在临床上较为常见, 给患者的健康带来了巨大的损害。研究外周神经损伤的类型及其相关信号通路的调控机制,为促进外周神经损伤后再生提供理论依据。目前对信号通路进行的研究提示,未来以信号通路为治疗方向可以解决外周神经损伤后再生的一些复杂过程。









 DownLoad:
DownLoad: