-
积雪草酸,是一种乌苏烷型三萜化合物,是积雪草中的主要药用成分,具有保肝、抗肿瘤、改善认知、降血糖、抗炎、抗菌、护肤等药理作用[1-2]。但是积雪草酸溶解度小,口服生物利用度低,较难通过血脑屏障,使得积雪草酸的使用受到一定限制,已有研究人员对积雪草酸进行了多种化学修饰,获得了一系列不同特性的积雪草酸衍生物[1-5]。由于积雪草酸结构的特殊性,化学修饰位点较少,生物修饰是对天然药物进行结构修饰的重要方法,已有研究报道通过微生物对积雪草酸进行生物修饰[6-8]。本实验通过总状共头霉CGMCC 3.2500对积雪草酸进行结构修饰,采用高分辨质谱(HR-ESI-MS)以及多种核磁共振(NMR)波谱:氢谱(1H NMR)、碳谱(13C NMR)、1H-13C异核单量子相干谱(1H-13C HSQC)、1H-13C异核多键相关谱(1H-13C HMBC)、1H-1H 核Overhauser效应谱(1H-1H NOESY)等技术对其结构进行确证,获得了一个新的化合物,为积雪草酸的应用与开发提供参考依据。
HTML
-
总状共头霉CGMCC 3.2500(中国普通微生物菌种保藏管理中心),4 ℃斜面保藏。
-
马铃薯培养基(PDA):将200 g马铃薯去皮切块置于1000 ml蒸馏水中,煮沸1 h,用纱布过滤,加葡萄糖20.0 g,琼脂15.0 g,将滤液补足至1.0 L,分装,121 ℃、30 min灭菌后,将试管倾斜放置,冷凝后即得固体马铃薯培养基。液体培养基的制备不加琼脂,其余步骤同上。
-
DRX-600光谱仪(德国Rheinstetten公司);Agilent 6538 VAD Accurate-Mass QTOF液质联用系统(美国安捷伦);Agilent 1260高效液相色谱仪(含G1311C 1260 VL型四元泵、G1316A 1260柱温箱、G1315D 1260 VL型全波长扫描检测器、G1364C 1260 FC-AS,美国安捷伦),ZORBAX Eclipse XDB-C18色谱柱;氘代吡啶(剑桥同位素实验室);积雪草酸(广西昌洲天然药业有限公司)。
-
将已经恢复培养的菌种接种至无菌的250 ml三角瓶(装有100 ml液体培养基)中,180 r/min、27 ℃下震荡培养72 h后取出,每瓶加入500 μl的积雪草酸乙醇溶液(4 mg/ml),相同条件继续培养10 d。设两组对照,一组接种微生物后只加入等体积的乙醇溶液(无底物);另一组加入等量的底物到空白培养基中,在相同条件下培养。发酵完成后过滤菌丝体,滤液用等体积的乙酸乙酯萃取3次,菌丝体用500 ml乙酸乙酯超声提取3次,每次30 min,合并乙酸乙酯萃取液及提取液,在60 ℃下真空浓缩至小体积,薄层色谱法(TLC)比较实验组与空白组的显色斑点,以氯仿/甲醇(9∶1)为展开剂,10%的硫酸乙醇溶液显色。
-
在10个1000 ml三角瓶中(每瓶装有400 ml培养基)以2%的接种量接入已恢复培养的总状共头霉CGMCC 3.2500菌种,置恒温振荡器中,180 r/min、27 ℃培养72 h后加入200.0 mg底物积雪草酸(溶于20 ml乙醇,每瓶加入2 ml),继续培养10 d。发酵完成后过滤菌丝体,滤液用等体积的乙酸乙酯萃取3次,将乙酸乙酯萃取液置旋转蒸发仪上浓缩至小体积,干燥,分别获得转化反应提取物0.4 g;转化反应提取物经凝胶柱纯化后,用半制备型高效液相色谱仪制备,流动相:甲醇/水/甲酸(60:40:0.05,V/V/V),流速3 ml/min,检测波长:210 nm,得到化合物1(10.5 mg,转化率5.25%)。
1.1. 材料
1.1.1. 菌株
1.1.2. 培养基
1.1.3. 试剂和仪器
1.2. 方法
1.2.1. 菌种的筛选
1.2.2. 扩大培养及产物分离
-
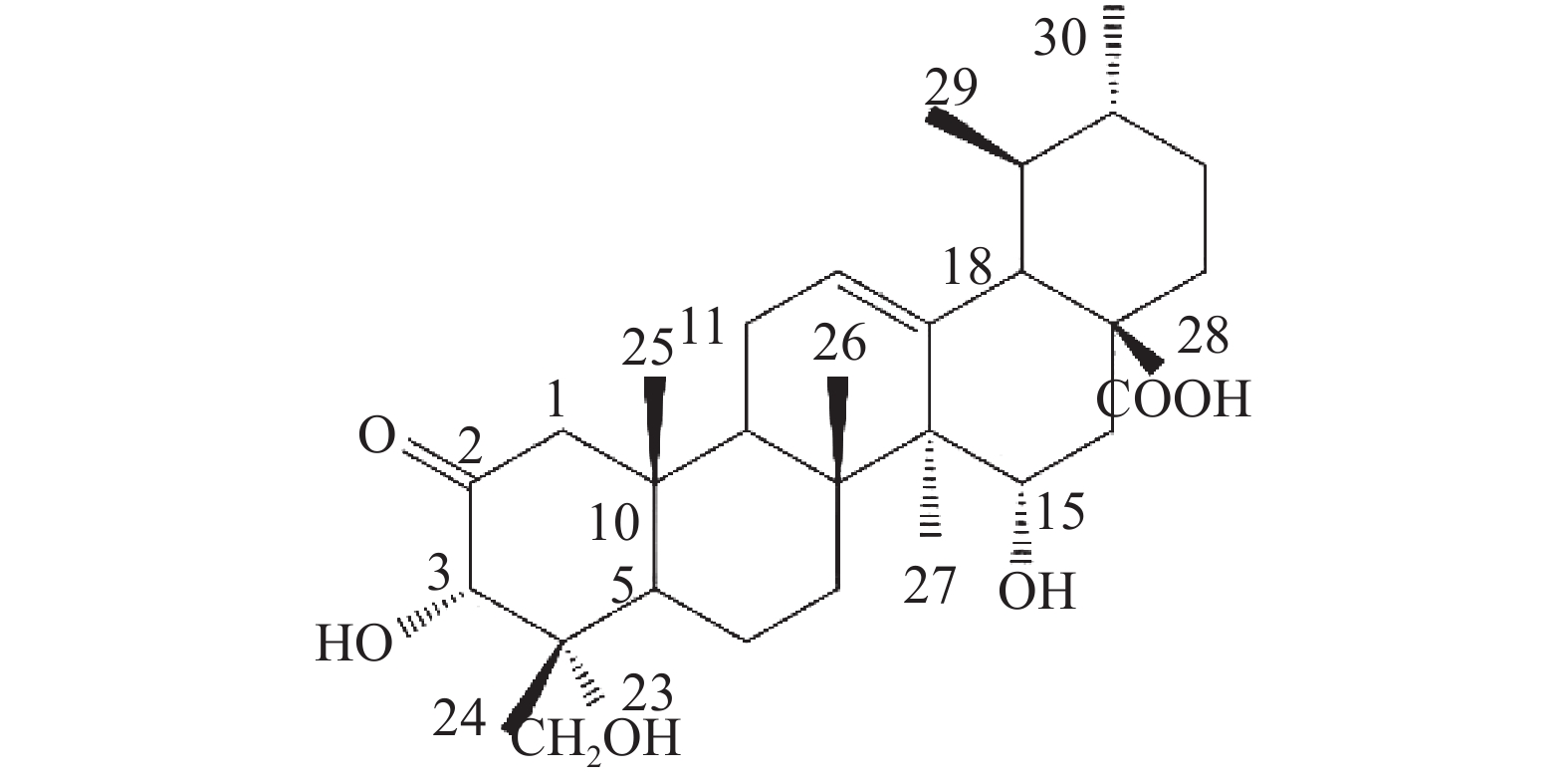
化合物1为白色固体粉末,高分辨质谱显示出[M-H]-分子离子峰m/z 501.3240,结合1H NMR谱和13C NMR谱推断确定分子式为C30H46O6。1H NMR(600 MHz, pyridine-d5)中,高场处有6组甲基氢信号δ 1.47 (3H, s)、δ 1.18 (3H, s)、δ 1.03 (3H, d, 6.6)、δ 0.98 (3H, s)、δ 0.92 (3H, d, 6.0)、δ 0.82 (3H, s);低场处有一活泼氢信号:δ 5.61 (t, 3.6),推测为双键上的氢。13C NMR谱检测结果显示两个不饱和碳原子δ 127.04和δ 140.86,一个羧基碳原子δ 180.36,一个羰基碳原子δ 213.29,综合以上信息可以推断该化合物为乌苏烷型五环三萜酸类化合物。
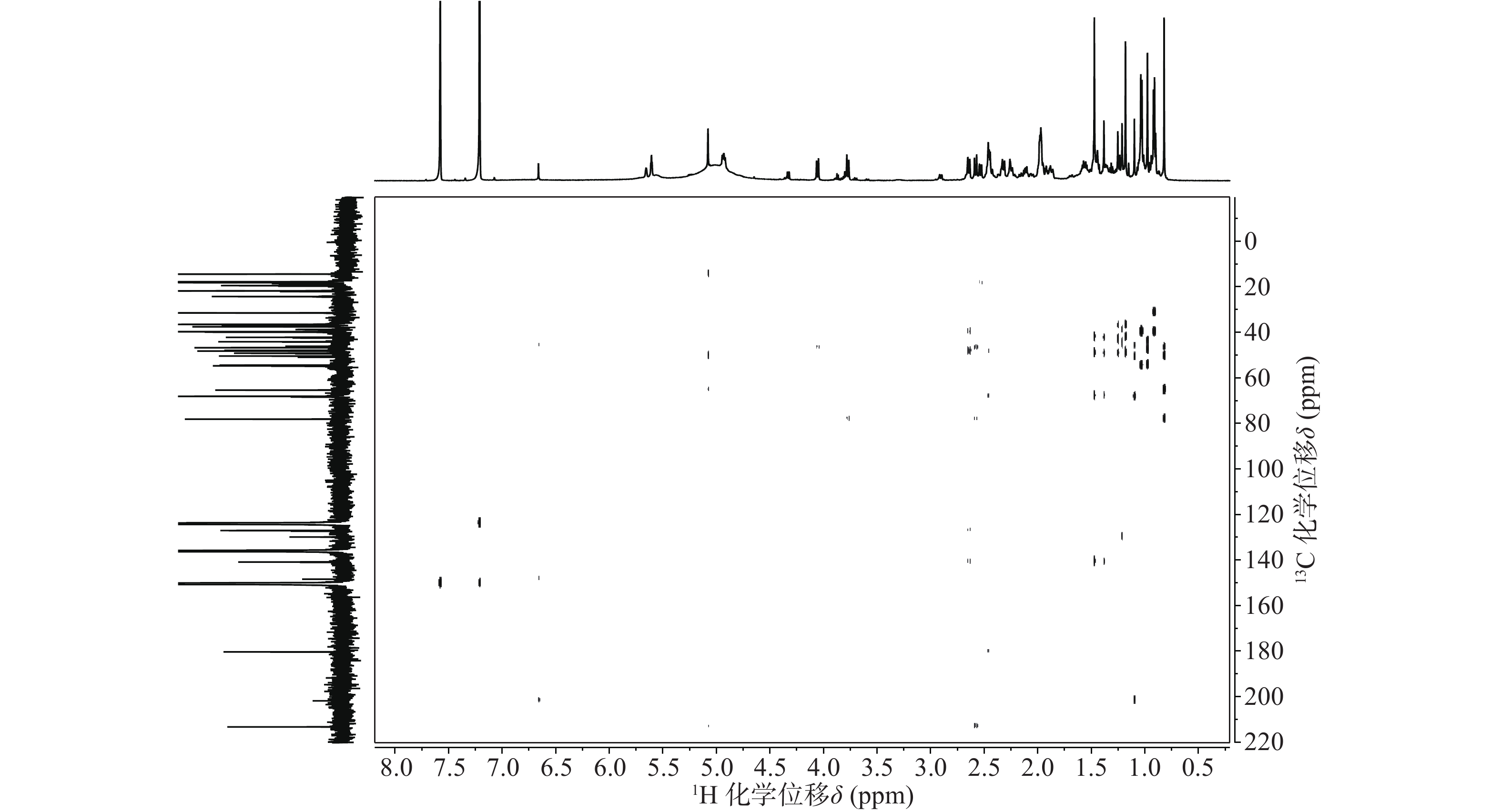
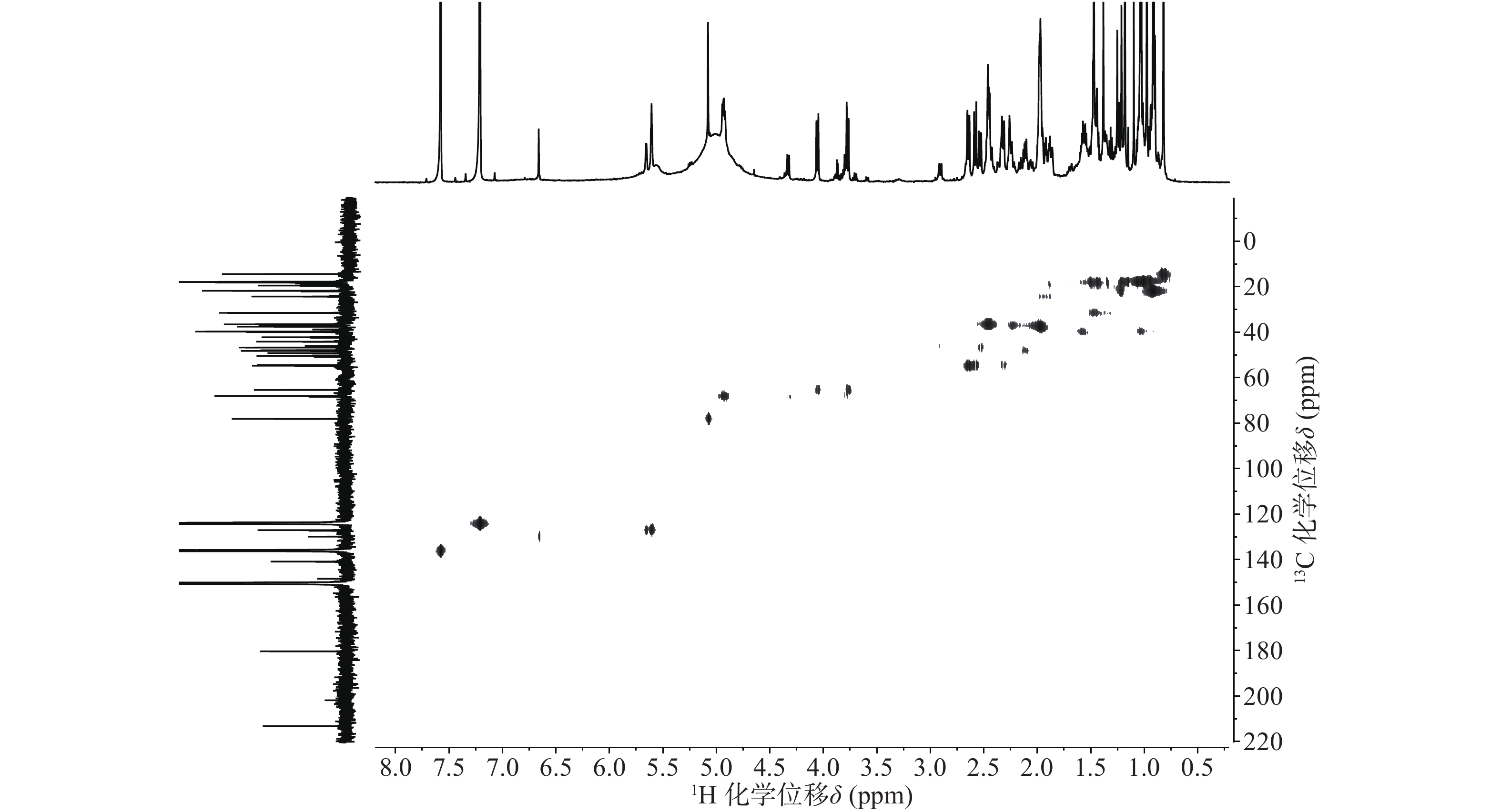
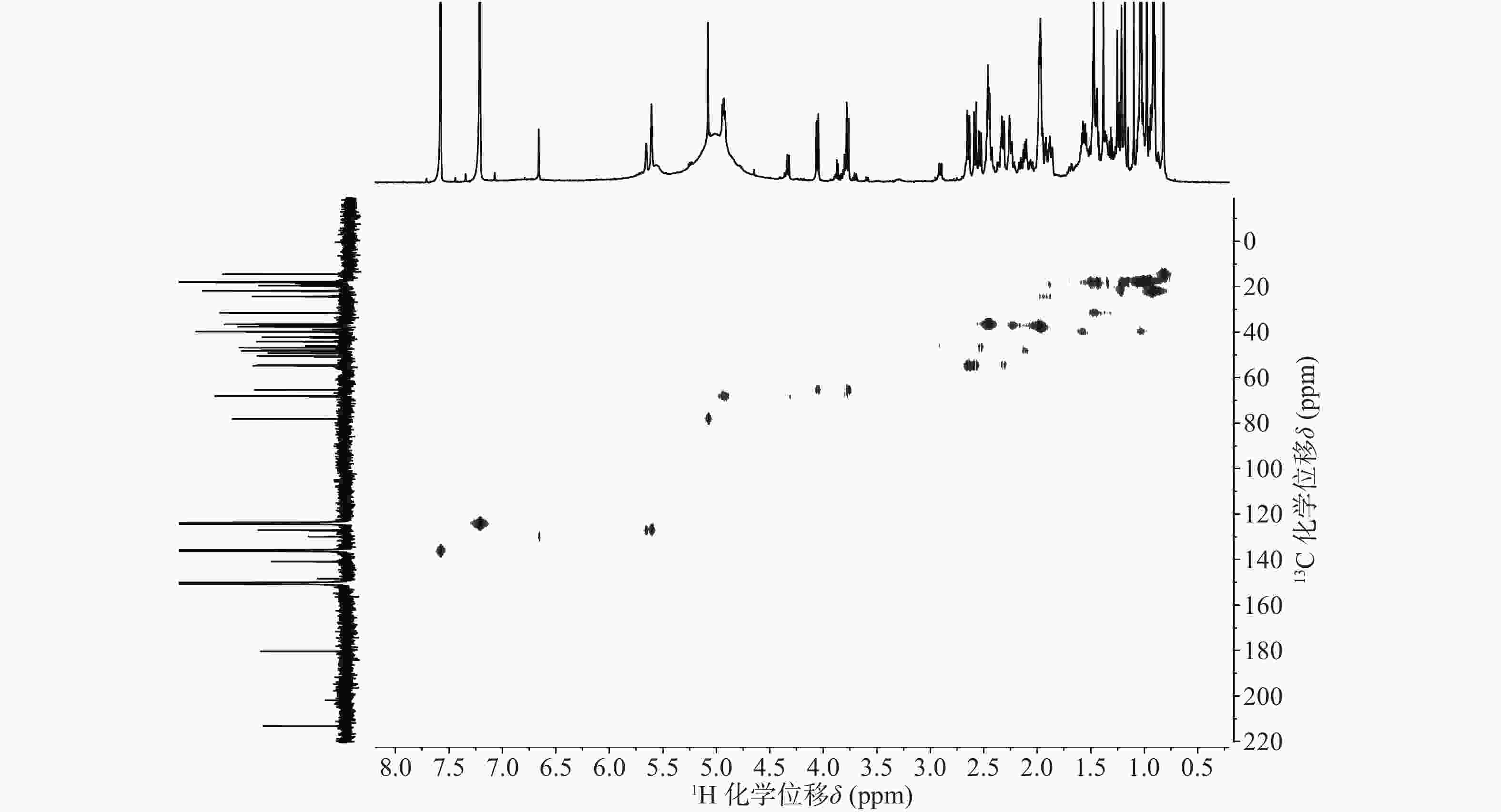
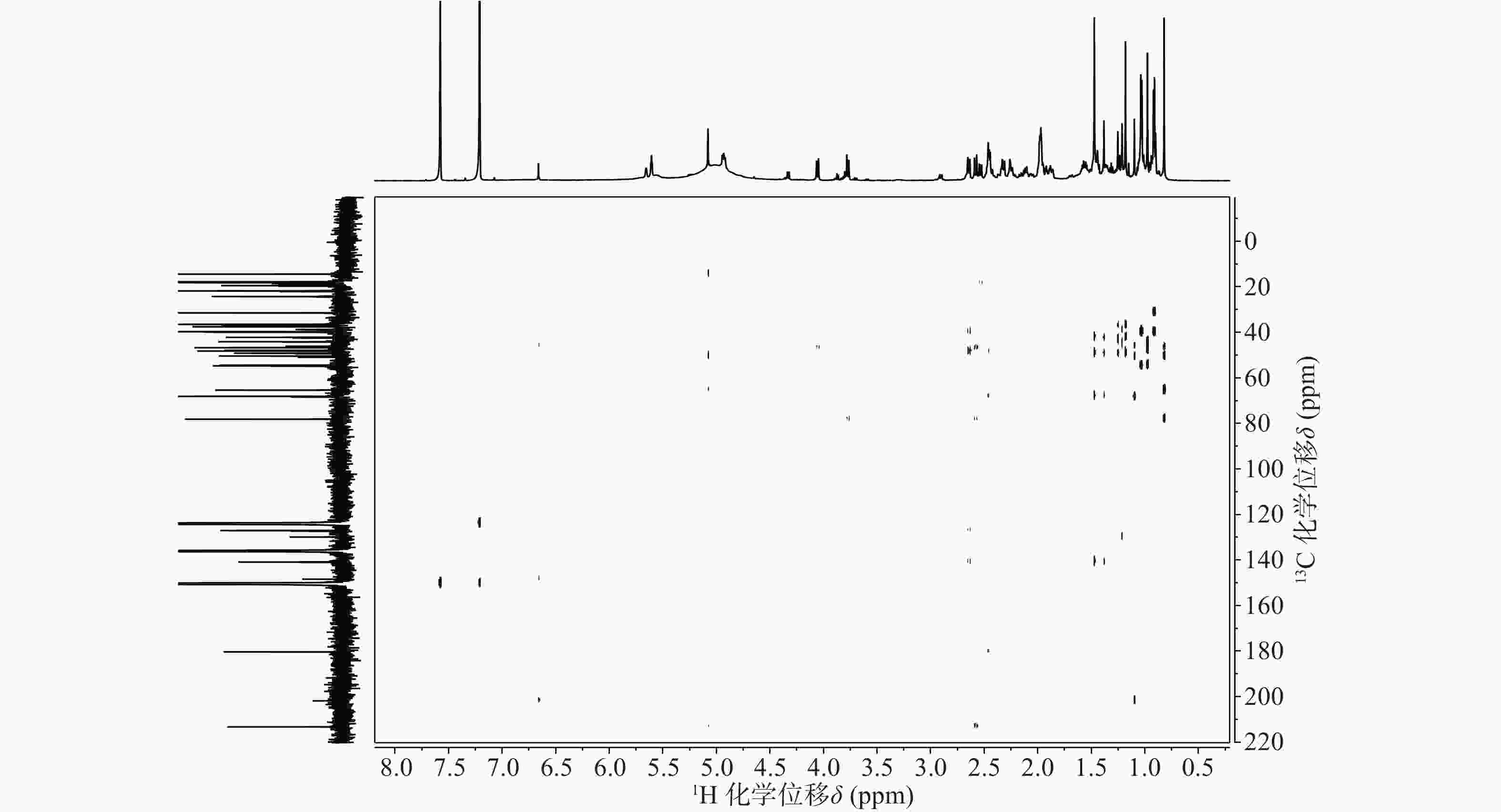
依据1H-13C HSQC谱(图1)、1H-13C HMBC谱(图2)可以对该化合物的碳氢作进一步的归属。
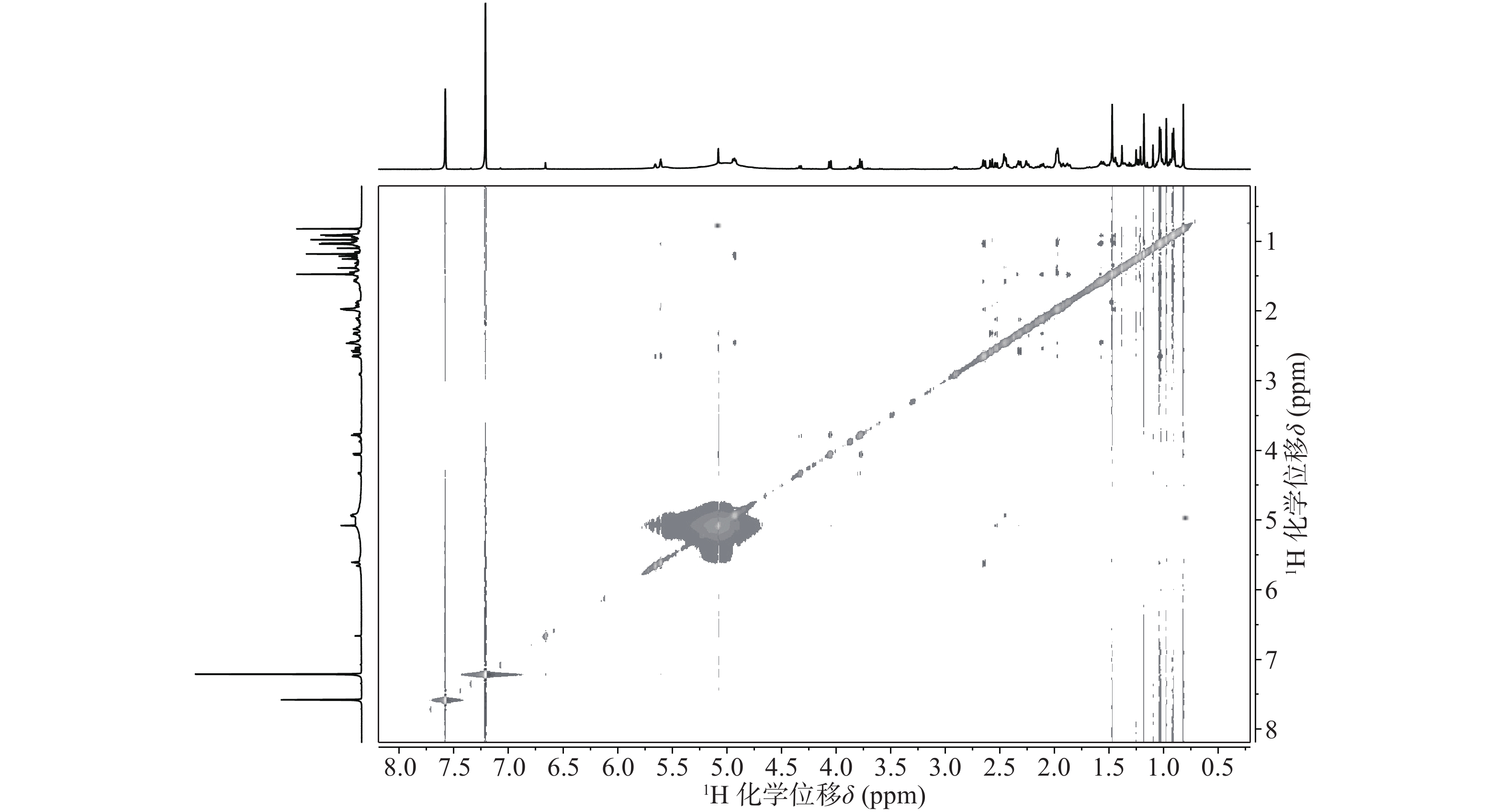
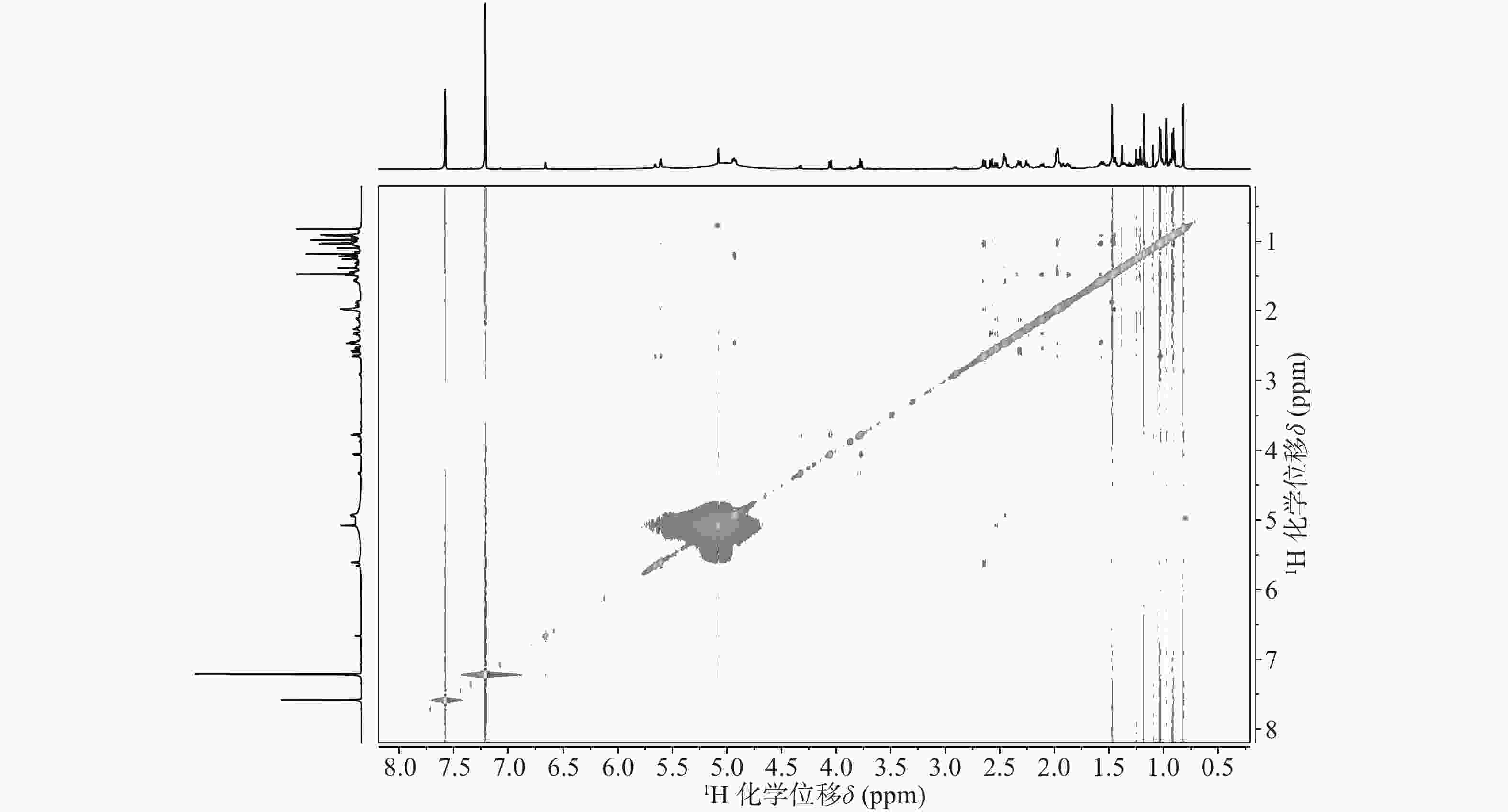
与底物积雪草酸比较,在13C NMR谱中,213.29 ppm出现一个羰基碳信号,该羰基碳与H-1 (δ 2.58, d, 12.0)、H-3 (δ 5.08, s) 远程相关,说明C-2位的羟基被氧化成羰基。H-3 (δ 5.08, s)和C-3 (δ 78.14)的化学位移未发生显著变化,但是在1H-1H NOESY(图3)中,可以观察到H-3 (δ 5.08, s)和H-24 (δ 0.82)之间的NOE效应,说明3位的羟基构型发生了变化,由β构型转变为α构型。
在13C NMR谱中,68.12 ppm处出现一个连氧碳信号,在1H NMR谱中,4.93 ppm(1H, dd, J=6.6, 9.6 Hz) 出现一个氢原子信号,二者在1H-13C HSQC谱中可观察到相关信号。在1H-13C HMBC谱中,可观察到H-27 (δ 1.47, s)、H-16 (δ 2.45, m)和δ 68.12的相关信号,说明15位发生了羟基化反应。同时H-15ax与H-16ax的耦合常数为9.6 Hz,确定该羟基为α型。
具体的1H NMR 和13C NMR 数据及归属见表1。
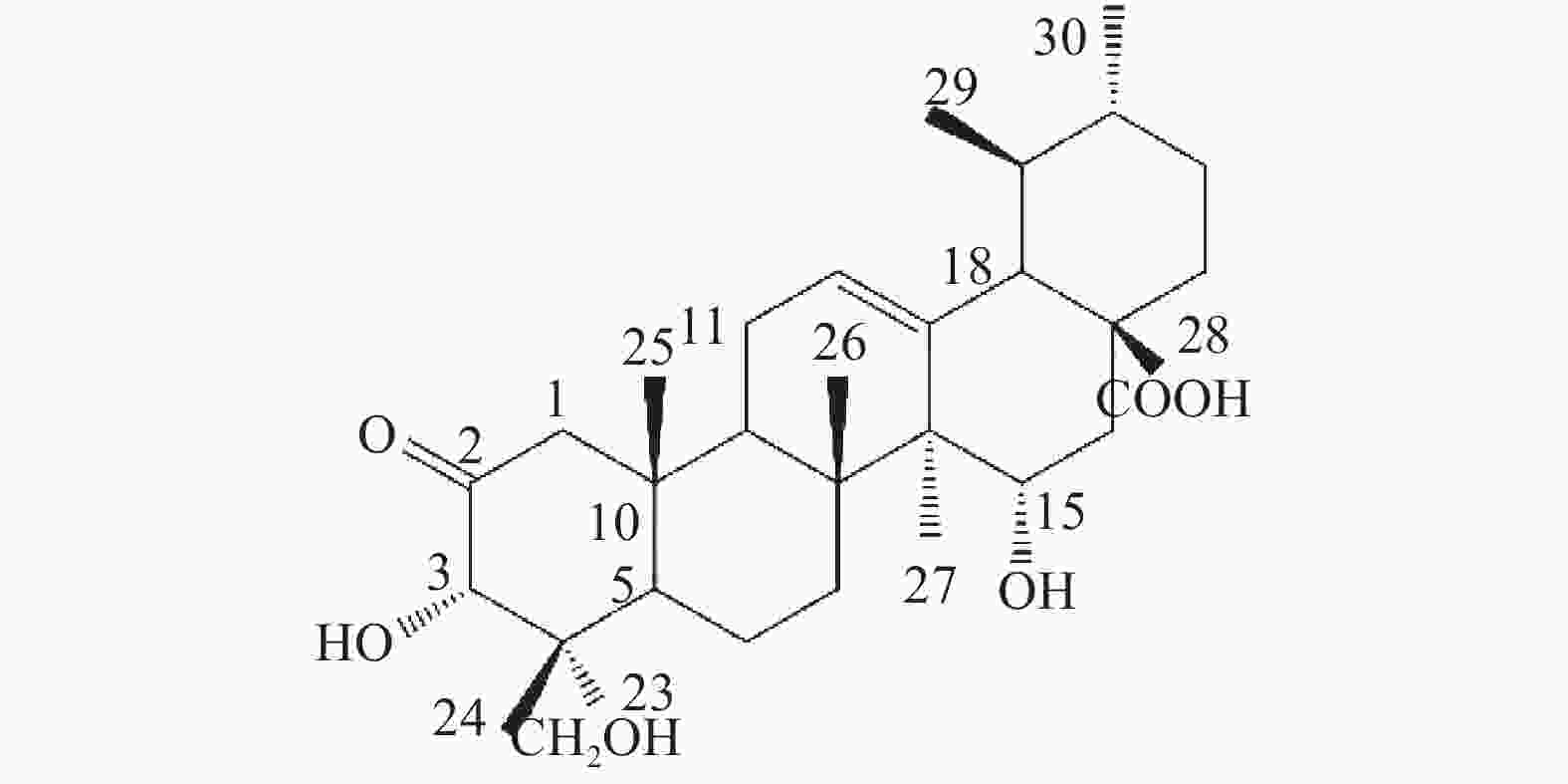
位点 C H HMBC 1 54.41 2.58 (d, 12.0); 2.31 (o) 2, 3, 5, 10, 25 2 213.29 / 3 78.14 5.08 (s) 2, 23, 24, 4 4 50.42 / 5 46.73 2.53 (d, 10.8) 4, 10, 7, 25, 24 6 19.48 1.87 (m), 1.97 (m) 7 36.52 2.46 (m); 2.33 (o) 8 42.19 / 9 48.22 2.12 (m) 1, 14, 8, 26 10 44.13 / 11 24.29 1.92 (t, 4.2); 1.96 (m) 8, 9, 13, 12 12 127.04 5.61 (t, 3.6) 14 13 140.86 / 14 49.18 / 15 68.12 4.93 (dd, 9.6, 6.6) 16 36.83 2.45 (m); 2.44 (m) 15, 18, 17, 28 17 47.89 / 18 54.70 2.64 (d, 11.4) 28, 13, 12, 17, 19, 22, 29 19 39.92 1.57 (m) 20 39.71 1.55 (m) 21 31.49 1.44 (m); 1.38 (s) 22 37.55 1.98 (m); 1.99 (m) 17, 20, 21, 28 23 65.38 4.06 (d, 11.4); 3.77 (d, 11.4) 3, 4, 5 24 14.43 0.82 (s) 3, 23, 4, 5 25 18.14 0.98 (s) 1, 5, 10 26 17.91 1.18 (s) 14, 8, 7 27 18.17 1.47 (s) 13, 15, 14, 8 28 180.36 / 29 17.78 1.03 (d, 6.6) 19, 20 30 21.79 0.92 (d, 6.0) 20, 21 综上,化合物1的结构确定为2-氧-3α,15α, 23-三羟基-12-烯-28-油酸(图4)。经文献检索,未发现与该化合物结构相同的报道,确定该化合物为新化合物。







 DownLoad:
DownLoad: