-
枇芩口服液是我院皮肤科的临床经验方,主要由枇杷叶、黄芩、赤芍、甘草等十三味中药制成,具有清热、祛湿、凉血等功效,临床主要用于治疗寻常型痤疮[1]。目前质量控制指标为制剂中的总黄酮以及君药黄芩中的成分[2],不能全面反映制剂的整体质量。组方中黄芩、枇杷叶为君药,具有清热解毒,凉血消肿作用。黄芩活性成分主要为黄酮类化合物,其中黄芩苷、黄芩素和汉黄芩素抗炎及抑菌效果好。枇杷叶等药材中含有绿原酸和咖啡酸成分,具有抗菌、消炎等作用。组方中的芍药苷及甘草中的甘草酸胺同样具有抗炎、抑菌及免疫调节等功效,诸药合用,在治疗寻常痤疮中发挥协同作用[3]。本研究拟采用双波长高效液相色谱法测定绿原酸、咖啡酸、芍药苷、黄芩苷、黄芩素、甘草酸铵和汉黄芩素含量[4-5],以进一步完善其质量标准。
-
Agilent 1200 Series高效液相色谱系统(含DAD检测器,美国Agilent公司);AUX220电子分析天平(日本岛津公司)。
-
枇芩口服液(自制,批号:20200220、20200224、20200315);黄芩苷对照品(批号:110715-201821,含量95.4%)、黄芩素对照品(批号:111595-200905,含量98.5%)、绿原酸对照品(批号:110753-201415,含量96.2%)、芍药苷对照品(批号:110736-201337,含量94.9%)、甘草酸铵对照品(批号:110731-201418,含量93.1%),咖啡酸对照品(批号:110885-200102)、汉黄芩素对照品(批号:111514-200403,含量98.0%)均购自中国食品药品检定研究院;乙腈(色谱纯,TEDIA公司,批号:18085030)。
-
Agilent Zorbax SB-C18(250 mm×4.6 mm,5μm)为色谱柱;流动相:0.02%磷酸水溶液(A)–乙腈(B)梯度洗脱,洗脱程序:0~10 min,10%B;10~20 min,10%~20%B;20~30 min,20%~25%B;30~40 min,25%~30% B;40~50 min,30%~40% B;50~60 min,40%~70% B; 60~65 min,70%~30% B;65~70 min,30%~10%B;流速:1.0 ml/min;柱温:35 ℃;进样量:20 μl。
检测波长:0~18.0 min,325 nm(检测绿原酸、咖啡酸);18.0~65.0 min,280 nm(检测芍药苷,黄芩苷、黄芩素、甘草酸铵、汉黄芩素)。
-
分别称取各对照品适量,精密称定,加甲醇制成含绿原酸355.9 μg/ml、咖啡酸550.0 μg/ml、芍药苷1480 μg/ml、黄芩苷772.7 μg/ml、黄芩素660.0 μg/ml、甘草酸铵642.4 μg/ml和汉黄芩素499.8 μg/ml的单一对照品储备液。精密吸取上述对照品储备液适量,置于同一10 ml的量瓶中,用甲醇稀释至刻度,摇匀,制得质量浓度依次为35.59、5.500、296.1、77.27、66.00、128.5和1.999 μg/ml的混合对照品溶液。
-
精密量取相应批号的枇芩口服液2ml,置10 ml量瓶中,加水稀释至刻度,摇匀,滤过,取续滤液即得。
-
根据处方中各药的比例,依次制备不含绿原酸(缺茵陈、墨旱莲和枇杷叶)、咖啡酸(缺夏枯草、墨旱莲、枇杷叶和丹参)、赤芍、黄芩和甘草的阴性样品,按“2.2.2”项下供试品溶液制备阴性溶液。
-
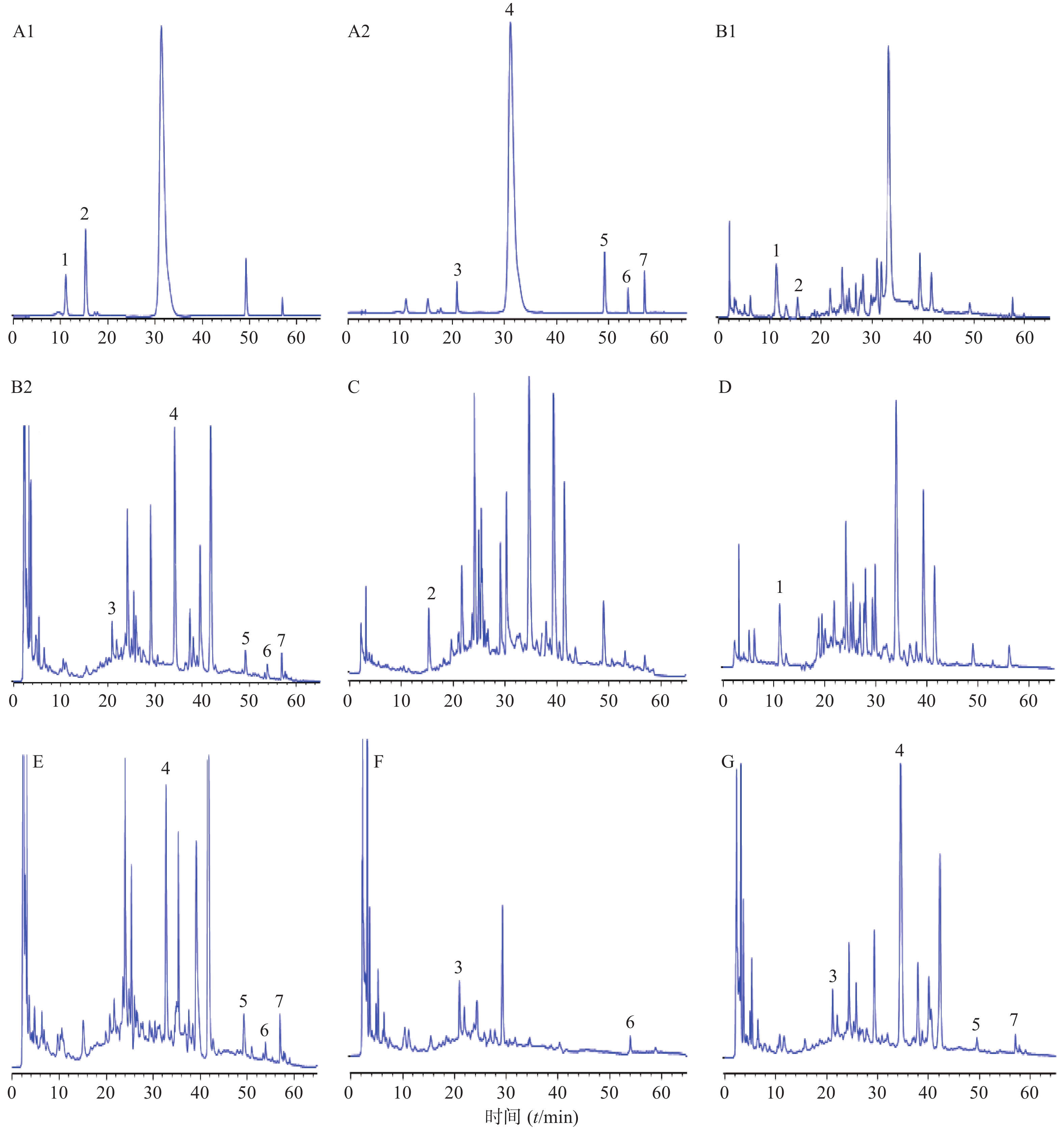
精密吸取混合对照品溶液、供试品溶液及阴性供试品溶液,按照“2.1”项下色谱条件测定,结果见图1。在供试品溶液色谱中,与对照品溶液色谱中绿原酸、咖啡酸、芍药苷、黄芩苷、黄芩素、甘草酸铵和汉黄芩素位置上均有相应的色谱峰出现,且色谱峰之间的分离度均˃1.5,理论板数˃3000。
-
分别精密吸取对照品储备液0.50、1.00、1.50、2.50、3.50、5.00ml置于5ml棕色量瓶中,加入甲醇稀释至刻度,摇匀。即得系列浓度的混合对照品溶液,按照上述的色谱分析条件进样分析,以浓度(X,μg/ml)为横坐标,峰面积(Y)为纵坐标,绘制标准曲线,得回归方程及线性范围,结果见表1。
有效成分 回归方程 r 线性范围(μg/ml) 绿原酸 Y=18.102X−0.0003 0.9991 3.559~35.59 咖啡酸 Y=51.805X+0.0874 0.9993 0.5500~5.500 芍药苷 Y=2.2865X+0.5859 0.9999 29.61~296.1 黄芩苷 Y=215.23X+43.349 0.9999 7.727~77.27 黄芩素 Y=14.843X−69.402 0.9998 6.600~66.00 甘草酸铵 Y=4.7447X−48.701 0.9995 12.85~128.5 汉黄芩素 Y=413.09X−64.950 0.9998 0.1999~1.999 -
精密量取“2.2.1”项下混合对照品溶液,按“2.1”项下色谱条件重复进样6次,记录峰面积。结果显示绿原酸、咖啡酸、芍药苷、黄芩苷、黄芩素、甘草酸铵和汉黄芩素峰面积的RSD分别为1.57%、1.98%、1.74%、1.07%、2.01%、1.41%和1.69%,表明仪器精密度良好。
-
取同一批样品(批号:20200224)6份,按照“2.2.2”项下方法制备供试品溶液,按“2.1”项下色谱条件进样测定峰面积,绿原酸、咖啡酸、芍药苷、黄芩苷、黄芩素、甘草酸铵和汉黄芩素峰面积的RSD分别为1.91%、1.90%、0.68%、1.41%、1.78%、1.40%和1.97%,表明方法重复性良好。
-
取同一份供试品溶液,分别于0、2、4、6、8、10 h,按“2.1”项下色谱条件进样测定峰面积,绿原酸、咖啡酸、芍药苷、黄芩苷、黄芩素、甘草酸铵和汉黄芩素峰面积的RSD分别为1.05%、1.90%、1.32%、1.06%、1.78%、2.02%和1.87%,结果表明供试品溶液在10 h内稳定。
-
精密量取已知含量(批号:20200224)的枇芩口服液1ml,共9份,分别按相当于绿原酸、咖啡酸、芍药苷、黄芩苷、黄芩素、甘草酸铵和汉黄芩素含有量的80%、100%、120%加入对照品溶液(不同加入量各3份),按“2.1”项下色谱条件进样,测定含量,并计算加样回收率和RSD,结果见表2。
成 分 样品含量
(µg)加入量
(µg)测得量
(µg)回收率
(%)平均回收率
(%)RSD
(%)绿原酸 66.46 56.94 122.4 98.24 98.55 0.75 66.46 71.18 136.2 98.02 66.46 88.98 154.9 99.39 咖啡酸 11.32 8.800 19.94 97.99 97.95 0.67 11.32 11.00 22.02 97.27 11.32 13.20 24.33 98.59 芍药苷 638.3 493.5 1117.7 97.14 97.58 0.67 638.3 592.2 1220.7 98.34 638.3 710.6 1329.3 97.25 黄芩苷 151.3 123.6 274.13 99.38 99.70 0.61 151.3 154.5 304.73 99.31 151.3 185. 5 337.47 100.40 黄芩素 103.7 84.48 187.93 99.71 98.88 1.51 103.7 105.6 209.07 99.78 103.7 132.0 231.93 97.15 甘草酸铵 115.5 102.8 217.17 98.90 98.26 1.41 115.5 128.5 243.0 99.22 115.5 154.2 264.57 96.67 汉黄芩素 1.908 1.599 3.427 95.02 96.41 1.41 1.908 1.999 3.837 96.50 1.908 2.399 4.252 97.72 -
取3个批号枇芩口服液(批号:20200220、20200224、20200315),按照“2.2.2”项下方法制备供试品溶液,按“2.1”项下色谱条件进样测定,计算7种成分的含量,结果见表3.
批号 绿原酸 咖啡酸 芍药苷 黄芩苷 黄芩素 甘草酸铵 汉黄芩素 20200220 69.54 12.11 615.3 157.3 99.97 112.2 1.709 20200224 66.46 11.32 638.3 151.3 103.7 115.5 1.908 20200315 65.80 11.21 633.2 149.2 105.2 117.2 1.911 -
试验中选择的7种成分来源于黄芩、夏枯草、茵陈、赤芍和甘草,全面覆盖君、臣、佐、使中的多味药材,将其作为质控指标,以提高质量控制的整体性和特征性。因测定成分绿原酸分别存在于组方中茵陈、枇杷叶等药材中,咖啡酸存在于枇杷叶、丹参等药材中,因此按照相应的方法制备阴性供试品溶液。
-
试验采用高效液相的DAD检测器分别对7种对照品在200~400 nm处扫描,结果绿原酸和咖啡酸在325 nm波长处有最大紫外吸收,芍药苷,黄芩苷、黄芩素、甘草酸铵、汉黄芩素在280 nm波长处有最大吸收,因此本实验选择325nm和280 nm作为测定波长。
-
考察了甲醇-0.1%磷酸水溶液、乙腈-水、乙腈-0.02%磷酸水溶液等流动相系统,结果采用乙腈-0.02%磷酸水溶液梯度洗脱,对照品溶液与供试品溶液各成分分离度较好,梯度洗脱中基线相对平稳,峰形较好。此外,对柱温、流速、进样量等色谱条件进行优化最终确定色谱条件。
Simultaneous determination of seven components in Piqin oral liquid by dual-wavelength HPLC
doi: 10.12206/j.issn.1006-0111.202008007
- Received Date: 2020-08-03
- Rev Recd Date: 2022-01-06
- Available Online: 2022-03-29
- Publish Date: 2022-03-25
-
Key words:
- Piqin oral liquid /
- HPLC /
- double-wavelength /
- content determination
Abstract:
| Citation: | WANG Qingfen, LIU Xiaoling, YANG Yuru, LI Jie. Simultaneous determination of seven components in Piqin oral liquid by dual-wavelength HPLC[J]. Journal of Pharmaceutical Practice and Service, 2022, 40(2): 161-164. doi: 10.12206/j.issn.1006-0111.202008007 |







 DownLoad:
DownLoad: