-
浅部真菌病是由致病真菌(皮肤癣菌、念珠菌、马拉色菌等)所致的浅表真菌感染,为皮肤科高发的感染性疾病,以足癣发病率最高,而趾间糜烂型和水疱型为最主要的发病类型。部队官兵作训任务重、居住环境艰苦,且常年穿着透气性较差的胶鞋、作战靴,战士间易交叉感染,是此类型疾病的高发人群。目前,足癣治疗方案为外用抗真菌药物,但临床上使用的多为单方制剂,对治疗合并有细菌感染伴有瘙痒症状的浅部真菌病效果较差,患者有时需使用两种或两种以上的药物,因携带不便,导致依从性较低。因此,本实验在前期研制复方酮康唑软膏的基础上[1],拟选用盐酸特比萘芬作为外用抗真菌药替代酮康唑,制备一种外用复方制剂——复方特比萘芬软膏,用于治疗浅部真菌皮肤病并发细菌感染,以解决酮康唑化学性质不稳定、易变色的问题,也方便部队官兵携带使用。同时,建立HPLC法同时测定该复方制剂的3个主要成分:特比萘酚、糠酸莫米松、莫匹罗星的含量。
HTML
-
1200型高效液相色谱仪(美国Agilent公司,DAD检测器);DV215CD型电子天平[奥豪斯仪器(上海)有限公司]。
-
盐酸特比萘芬对照品(批号:100563-201402,含量99.8%)、莫匹罗星对照品(批号:130568-200501,含量94.2%)、糠酸莫米松对照品(批号:100930-201201,含量99.9%)均购自中国食品药品检定研究院。盐酸特比萘芬原料药(批号:20150405)、莫匹罗星原料药(批号:20150301)购自武汉鑫佳灵生物科技有限公司;糠酸莫米松原料药(批号:20150228,浙江仙琚制药股份有限公司)。聚乙二醇400、聚乙二醇3350(中国医药对外贸易公司),甲醇为色谱纯,水为超纯水;其余试剂均为分析纯。
1.1. 仪器
1.2. 试药
-
精密称定盐酸特比萘芬对照品10.2 mg,置10 ml量瓶中,用70%甲醇稀释至每1 ml含盐酸特比萘芬1020 μg的对照品储备液。精密移取盐酸特比萘芬对照品储备液1 ml置10 ml量瓶中,加70%甲醇稀释至刻度,摇匀,即得含量为102 μg/ml的盐酸特比萘芬对照品溶液。
-
精密称定莫匹罗星20.2 mg,置10 ml量瓶中,用70%甲醇稀释至每1 ml含莫匹罗星2020 μg的对照品储备液。再精密移取莫匹罗星的对照品储备液1 ml置10 ml量瓶中,加70%甲醇稀释至刻度,摇匀,即得含量为202 μg/ml的莫匹罗星对照品溶液。
-
精密称定糠酸莫米松10.1 mg,置100 ml量瓶中,用70%甲醇稀释至每1 ml含糠酸莫米松101 μg的对照品储备液。再精密移取糠酸莫米松的对照品储备液1 ml置10 ml量瓶中,加70%甲醇稀释至刻度,摇匀,即得含量为10.1 μg/ml的糠酸莫米松对照品溶液。
-
分别取盐酸特比萘芬、莫匹罗星和糠酸莫米松对照品储备液各1 ml,置10 ml量瓶中,加70%甲醇稀释至刻度,摇匀,即得。
-
精密称取原料药盐酸特比萘芬1 g、莫匹罗星2 g、糠酸莫米松0.1 g,置100 ml量瓶中,用70%甲醇稀释至刻度,摇匀,作为供试品,同法制备3份。精密吸取上述溶液1 ml置100 ml量瓶中,加70%甲醇稀释至刻度,摇匀,即得供试品溶液。
-
按“2.1.5”项下,分别配制缺盐酸特比萘芬、缺莫匹罗星、缺糠酸莫米松成分的溶液,即得阴性对照溶液。
-
色谱柱为ZORBAX SB-C8柱(250 mm×4.6 mm,5 µm),流动相为甲醇-0.1%磷酸溶液(70∶30),检测波长248 nm,流速1.0 ml/min,柱温30 ℃,进样量10 µl。
-
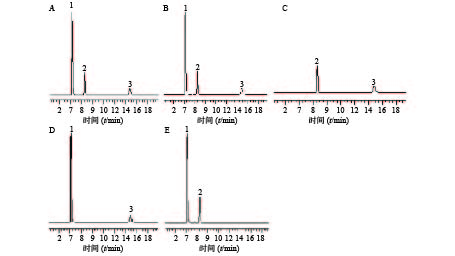
分别取供试品溶液、对照品溶液及阴性对照溶液适量,滤过,取续滤液10 μl注入HPLC仪,分别记录色谱图。实验结果表明,该方法专属性良好,阴性对照无干扰。结果见图1。
-
分别精密吸取盐酸特比萘芬、莫匹罗星和糠酸莫米松对照品储备液各0.2、0.4、0.8、1.0、1.2、1.4、1.6、2 ml置10 ml量瓶中,用70%甲醇稀释配制成含盐酸特比萘芬浓度为20.4、40.8、81.6、102.0、122.4、142.8、163.2、204.0 µg/ml,含莫匹罗星浓度为40.4、80.8、161.6、202.0、242.4、282.8、323.2、404.0 µg/ml,含糠酸莫米松浓度为2.02、4.04、8.08、10.10、12.12、14.14、16.16、20.20 µg/ml的混合对照品溶液,滤过,取续滤液10 μl,按“2.2”项下色谱条件分析,记录色谱图,计算峰面积。以峰面积A对对照品浓度C(µg/ml)线性回归。实验结果表明,盐酸特比萘芬在20.4~204.0 µg/ml浓度范围内线性关系良好,回归方程为A=1.905×10C+25.90,r=0.999 7;莫匹罗星在40.4~404.0 µg/ml浓度范围内线性关系良好,回归方程为A=2.440C+1.446,r=0.999 8;糠酸莫米松在2.02~20.20 µg/ml浓度范围内线性关系良好,回归方程为A=2.838×10C+15.12,r=0.999 7。
-
精密吸取同一份混合对照品溶液6次,每次10 μl,分别注入HPLC仪,记录峰面积,计算RSD值。盐酸特比萘酚、莫匹罗星、糠酸莫米松RSD(n=6)分别为0.05%、0.12%、0.25%,实验结果表明仪器精密度良好。
-
精密称取同一批供试品6份,按“2.1.5”项下方法制备供试品溶液,滤过,取续滤液按“2.2”项下色谱条件分析,分别记录色谱图,测定峰面积,计算含量。盐酸特比萘酚、莫匹罗星、糠酸莫米松RSD(n=6)分别为0.19%、0.08%、0.44%,实验结果表明,RSD均小于1%,方法重复性良好。
-
取供试品溶液,分别于0、2、4、6、8、12、24 h取样10 μl注入HPLC仪,测定并计算不同时间点莫匹罗星、糠酸莫米松和盐酸特比萘芬的含量。盐酸特比萘酚、莫匹罗星、糠酸莫米松RSD(n=6)分别为0.66%、0.77%、0.57%。结果表明,供试品溶液在24 h内稳定。
-
分别精密量取“2.1.1”、“2.1.2”、“2.1.3”项下盐酸特比萘芬、莫匹罗星和糠酸莫米松的对照品储备液各0.8、1、1.2 ml,分别置于3个10 ml量瓶中,加70%甲醇稀释至刻度,摇匀,得低、中、高3种浓度的混合对照品溶液,每个浓度平行配制3份。滤过,弃去初滤液,取续滤液10 μl注入HPLC仪,测定并计算。实验结果表明,盐酸特比萘芬、莫匹罗星和糠酸莫米松平均回收率分别为(99.39±0.82)%、(99.21±0.59)%、(99.97±0.81)%,说明此方法回收率符合要求。
-
按“2.1.5”项下配制供试品3批,每批3份,精密称取供试品1 ml,置于100 ml量瓶中,加70%甲醇稀释至刻度,摇匀,得供试品溶液9份,测定,外标法计算药物含量。结果见表1。
供试品 莫匹罗星 糠酸莫米松 特比萘芬 1 99.32±0.26 100.8±0.37 100.2±0.38 2 99.95±0.26 101.2±0.52 101.8±0.56 3 100.3±0.87 101.4±0.72 100.5±1.04
2.1. 溶液的制备[1]
2.1.1. 盐酸特比萘芬对照品溶液的制备
2.1.2. 莫匹罗星对照品溶液的制备
2.1.3. 糠酸莫米松对照品溶液的制备
2.1.4. 混合对照品溶液的制备
2.1.5. 供试品溶液的制备
2.1.6. 阴性对照溶液的制备
2.2. 色谱条件
2.3. 专属性试验
2.4. 标准曲线制备及线性关系考察
2.5. 精密度试验
2.6. 重复性试验
2.7. 稳定性试验
2.8. 回收率试验
2.9. 样品含量测定结果
-
紫外光谱扫描结果显示,盐酸特比萘芬在282 nm具有最大吸收峰,莫匹罗星在220 nm处具有最大吸收峰,糠酸莫米松在248 nm处具有最大吸收峰。由于3个组分中糠酸莫米松含量较低,仅占处方量的0.1%,而盐酸特比萘芬占1%,莫匹罗星占2%。故为了让这3种药物能同时测定并保证糠酸莫米松的响应值,确定盐酸特比萘芬、莫匹罗星和糠酸莫米松的检测波长为248 nm,在此波长下3种主药均有较好的响应值,建立的HPLC法回收率试验结果符合要求。
-
笔者曾首先尝试以甲醇-pH5.5磷酸盐缓冲液(65∶35)作为流动相,色谱柱选用C18柱[2],结果显示该色谱条件下,盐酸特比萘芬出峰时间太长,而莫匹罗星及糠酸莫米松的出峰时间偏早,且杂峰较多;还曾尝试以甲醇-水、甲醇-醋酸铵、甲醇-乙腈-水、乙腈-水等、甲醇-0.1%磷酸溶液、四氢呋喃-乙腈-四甲基氢氧化铵缓冲液[3-10]作为流动相,试验结果表明,甲醇-0.1%磷酸溶液(70∶30)作为流动相,C8色谱柱的条件下,盐酸特比萘芬出峰时间明显提前,三大主药出峰时间都在20 min以内,基线平稳,各峰分离度良好;但该条件下盐酸特比萘芬的峰型对称性不佳,有拖尾现象。故在此基础上,考虑莫匹罗星(弱酸性)[11-12],实验又尝试通过调节流动相pH改善峰型,发现在pH7.5时,盐酸特比萘芬具有良好的对称性,但莫匹罗星与糠酸莫米松拖尾严重。为保证大部分主药的峰型良好,本课题最终选用色谱条件为C8色谱柱,甲醇-0.1%磷酸溶液(70∶30)作为流动相。实验采用HPLC法,建立同时测定盐酸特比萘芬、莫匹罗星和糠酸莫米松的含量测定方法,所建立的分析方法简便、准确、灵敏度高,阴性对照无干扰。







 DownLoad:
DownLoad: