-
放线菌是天然药用活性分子的重要源头[1]。然而,近年陆生放线菌来源化合物的重复发现率日趋增高,海洋来源放线菌因其特殊的生长环境和丰富的次生代谢产物越发引起关注[1-2]。
海绵作为代表性的海洋底栖共生生物体,是产生功能分子的重要源头[3]。而越来越多的证据表明共附生微生物是海绵化学多样性的重要来源[4-6]。因此,开展海绵共附生微生物化学成分的研究,寻找新颖的、具有活性功能的小分子化合物显得十分必要。
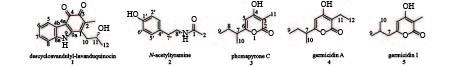
课题组从海绵共附生放线菌Streptomyces sp. LHW2432发酵物中发现了2个生物碱类化合物1和2,以及3个吡喃酮类化合物3~5(图1)。其中,1为新型天然产物,具有抑制耐甲氧西林金黄色葡萄球菌(MRSA)和耻垢分支杆菌的微弱活性,并可作为合成神经细胞保护剂三环咔唑类生物碱的关键前体[7]。
HTML
-
Agilent 600 MHz 核磁共振仪(Agilent);Xevo G2-XS QTOF质谱仪(Waters);Acquity UPLC 高效液相色谱仪(Waters);Interchim PuriFlash 450中压色谱仪(Interchim);ODS(YMC,C18,21.2 mm × 250 mm,5 μm);XBridge C18半制备型液相色谱柱(Waters, XBridge Prep C18,10 mm × 250 mm,5 μm);C18制备型液相色谱柱(Phenomenex,Luna C18,21.2 mm × 250 mm,5 μm);N-1000型旋转蒸发仪(上海爱郎);SK5200H型超声仪(上海科导);振荡培养箱(上海知楚);培养箱(上海博远)。色谱级溶剂(Merk);分析纯试剂(上海化学试剂公司);氘代试剂(Cambridge Isotope Laboratories)。
-
TSBY培养基:胰蛋白胨大豆肉汤(30 g/L)、酵母提取物(5 g/L)、蔗糖(100 g/L)、消泡剂(1 ml/L);SFM培养基:低温黄豆饼粉(20 g/L)、琼脂(20 g/L)、甘露醇(20 g/L),pH 7.2~7.4;MH培养基(Solarbio®):MH肉汤(22 g/L);PDA培养基:马铃薯(200 g/L)、琼脂(20 g/L)、葡萄糖(20 g/L)。
-
链霉菌LHW2432分离自南海海绵(红色指状,种属未鉴定,本实验室编号1524),经16S rRNA基因序列(1376 bp)比对,与Streptomyces purpurascens相似度达99.42%,鉴定为链霉菌。
指示菌蕈状芽胞杆菌(Bacillus mycoides)、耐甲氧西林金黄色葡萄球菌(Methicillin-resistant Staphylococcus aureus CICC10201)、耻垢分支杆菌(Mycobacterium smegmatis mc2155)、大肠杆菌(Escherichia coli)和白色念珠菌(Candida albicans)均取自上海交通大学医学院仁济医院药学部海洋药物研究中心菌种库。
-
将LHW2432接种于SFM平板,30 ℃培养5 d,挑取单菌落接种于装有10 ml TSBY培养基的100 ml三角瓶中(加有不锈钢弹簧),30 ℃,220 r/min培养3 d,作为一级种子液;一级种子液以1∶20(V/V)接种于装有150 ml TSBY培养基的500 ml三角瓶中(加有不锈钢弹簧),30 ℃,220 r/min培养3 d,该发酵液作为二级种子液,继续同样条件的发酵,最终得到12 L发酵液。用等体积乙酸乙酯萃取发酵液3次,萃取液浓缩悬干后得到11.3 g浸膏。
-
通过正相硅胶柱色谱分离粗浸膏,二氯甲烷-甲醇洗脱梯度:150∶1、100∶1、80∶1、50∶1、25∶1、15∶1、5∶1、1∶1,共得到13个馏分A~M。合并F与G馏分(1.91 g干重)后经中压ODS色谱柱继续分离[乙腈-水(0.1%甲酸):5%~95%梯度洗脱,6 h,15 ml/min],得到16个馏分FG1~FG16。
化合物1(3.1 mg)和4(7.7 mg)由FG12(42.3 mg干重)馏分经制备型HPLC分离获得,洗脱条件为:9 ml/min,53%甲醇-水(0.1%甲酸)。
化合物3(9.0 mg)和5(2.1 mg)由FG10(37.4 mg干重)馏分经制备型HPLC分离获得。FG10经洗脱[9 ml/min,42%甲醇-水(0.1%甲酸)]得到化合物3和组分FG10-5(7.5 mg干重);组分FG10-5进一步洗脱得到化合物5,洗脱条件为:3 ml/min,30%乙腈-水(0.1%甲酸)。
化合物2(121 mg)分离自馏分J。J馏分(4.357 g干重)经凝胶柱色谱[流动相:二氯甲烷-甲醇(1∶1)]砍断得到5个亚馏分J1~J5。选取J4(1.112 g干重)再经中压ODS柱色谱[甲醇-水(0.1%甲酸):10%~95%梯度洗脱,2 h,15 ml/min]分离获得化合物2。
-
如表1所示,将200 μl~2 ml过夜生长的5种指示菌的菌液加入水解酪蛋白琼脂培养基(MHA)或马铃薯葡萄糖琼脂培养基(PDA)中(约50 ℃)稀释,摇匀后倒入培养皿中。待凝固后,滴加溶解于3 μl DMSO溶液的样品(约10 μg),设置一个阳性对照和一个DMSO阴性对照,平板于相应条件培养12 h后观察抑菌圈(表1)。
指示菌 培养条件 阳性药 B. mycoides(蕈状芽胞杆菌) MHA, 37 ℃ 万古霉素 S. aureus(金黄色葡萄球菌) MHA, 37 ℃ 万古霉素 M. smegmatis(耻垢分支杆菌) MHA, 37 ℃ 卡那霉素 E. coli(大肠杆菌) MHA, 37 ℃ 萘啶酮酸 C. albicans(白色念珠菌) PDA, 30 ℃ 两性霉素 -
使用肉汤稀释法进行96孔板实验[8]。样品和阳性对照药分别设置3个平行组,先将过夜培养的指示菌用MH肉汤培养基进行1 000倍稀释后加入96孔板,每孔50 μl。第1列至第10列样品浓度依次为128、64、32、16、8、4、2、1、0.5、0.25 μg/ml。加样后37 ℃培养18 h,通过分光光度计(A600)检测菌液澄清度。最后,根据实验结果调整测试药物浓度梯度,重复实验。
1.1. 仪器和材料
1.1.1. 实验仪器和试剂
1.1.2. 培养基
1.2. 菌株来源及鉴定
1.3. 发酵与萃取
1.4. 提取分离
1.5. 抗菌活性测试方法
1.5.1. 平板涂布法
1.5.2. 微量稀释法
-
化合物1:红棕色固体。ESI-MS m/z 270.1133 [M+H]+(calcd for C16H16NO3,270.112 5),提示其相对分子质量为269,推测该化合物结构中含有一个N原子,结合1H-NMR和13C-NMR确定其分子式为C16H15NO3,计算其不饱和度为8。1H-NMR在低场区显示出4个芳烃质子信号δH 7.85 (1H,m)、7.51 (1H,m)、7.23 (2H,dd,J = 6.0,3.1 Hz),高场区显示2个甲基质子信号δH 1.92 (3H,s)和1.25 (3H,d,J = 6.1 Hz),1个亚甲基质子信号δH 2.77 (2H,m),1个连氧次甲基质子信号δH 3.96 (1H,dt,J = 8.0,6.1 Hz);13C-NMR和DEPT谱,在低场区显示出2个羰基碳信号 (δC 172.8,183.6)、4个芳基次甲基碳信号 (δC 113.4,120.2,123.9,124.1),6个芳基或双键季碳信号 (δC 110.9,125.7,134.8,137.1,139.8,146.5),在高场区显示出2个甲基碳信号 (δC 12.2,23.8)、1个亚甲基碳信号 (δC 37.8) 和1个连氧次甲基碳信号 (δC 65.9);将波谱信号进行归属,1H-NMR (600 MHz,DMSO-d6):δ 7.85 (m,H-5),7.51 (m,H-8),7.23 (dd,J = 6.0,3.1 Hz,H-6,7),3.96 (dt,J = 8.0,6.1 Hz,H-11),2.76 (m,H-10),1.92 (s,H-13),1.25 (d,J = 6.1 Hz,H-12)。13C NMR (151 MHz,DMSO-d6):δ 183.6 (C-3),172.8 (C-4),146.5 (C-9a),139.8 (C-1),137.1 (C-8a),134.8 (C-2),125.7 (C-4b),124.1 (C-7),123.9 (C-6),120.2 (C-5),113.4 (C-8),110.9 (C-4a),65.9 (C-11),37.8 (C-10),23.8 (C-12),12.2 (C-13)。化合物数据与文献[9-10]报道一致,结构鉴定为咔唑-3,4-邻醌生物碱descycloavandulyl-lavanduquinocin。
化合物2:白色无定型粉末。ESI-MS m/z 180.1022 [M+H]+(calcd for C10H14NO2,180.101 9),相对分子质量为179,说明该化合物可能含有一个N原子,结合13C-NMR确定其分子式为C10H13NO2,计算不饱和度为5。1H-NMR中包含4个芳烃质子信号δH 6.97 (2H,m)、6.67 (2H,m),结合13C-NMR中的6个双键碳信号,推断该化合物中含有一个对位二取代的苯环,其中一个碳信号 (δC 155.6) 化学位移值向低场偏移,说明是一个连氧取代的芳基碳原子;归属该化合物的波谱信号,1H-NMR (600 MHz,DMSO-d6):δ 9.20 (brs,1′-OH),7.87 (t,J = 5.5 Hz,1-NH),6.97 (m,H-3′,H-5′),6.67 (m,H-2′,H-6′),3.17 (m,H-8′),2.56 (dd,J = 8.4,6.6 Hz,H-7′),1.77 (s,H-2);13C NMR (151 MHz,DMSO-d6):δ 169.1 (C-1),155.6 (C-1′),129.6 (C-4′),129.5 (C-1′,C-5′),115.1 (C-2′,C-6′),40.6 (C-8′),34.5 (C-7′),22.7 (C-2)。与文献数据[11-12]比对,该化合物被鉴定为N-乙酰酪胺(N-acetyltyramine)。
化合物3:淡黄色固体。ESI-MS m/z 183.1028 [M+H]+(calcd for C10H15O3,183.101 6),提示其相对分子质量为182,结合1H和13C-NMR数据推测其分子式为C10H14O3,不饱和度为4。1H-NMR的低场区显示出一个双键质子信号δH 5.95 (1H,s),高场区显示3个甲基质子信号δH 0.81 (3H,t,J = 7.4 Hz)、1.10 (3H,d,J = 6.9 Hz) 和1.73 (3H,s),1对亚甲基质子信号δH 1.54 (1H,m)、1.44 (1H,m),1个次甲基质子信号δH 2.43 (1H,m);13C-NMR和DEPT谱显示共有10个碳信号,包括4个双键或羰基季碳信号,1个sp2杂化的双键次甲基碳信号,1个sp3杂化的次甲基碳信号,1个sp3杂化的亚甲基碳信号,3个甲基碳信号。如下为化合物的波谱信号归属,1H-NMR (600 MHz,DMSO-d6):δ 5.95 (s,H-5),2.43 (m,H-7),1.73 (s,H-11),1.54 (m,H-8a),1.44 (m,H-8b),1.10 (d,J = 6.9 Hz,H-10),0.81 (t,J = 7.4 Hz,H-9)。13C NMR (151 MHz,DMSO-d6):δ 165.8 (C-2),165.5 (C-4),165.2 (C-6),98.9 (C-5),96.2 (C-3),38.7 (C-7),26.9 (C-8),17.7 (C-10),11.3 (C-9),8.5 (C-11)。结合比对文献[13]核磁谱图数据,该化合物鉴定为phomapyrone C。
化合物4:棕色固体。ESI-MS m/z 197.1181 [M+H]+(calcd for C11H17O3,197.1172),提示其相对分子质量为196,结合1H和13C-NMR数据推测其分子式为C11H16O3,不饱和度为4。比较化合物4和3的核磁数据,发现4的1H-NMR低场区存在一个双键质子信号δH 5.95 (1H,s),13C-NMR 低场区存在4个双键或羰基季碳信号 (δC 165.8,165.2,164.7,102.5)、1个双键次甲基碳信号 (δC 98.8),这些核磁数据与化合物3的吡喃-2-酮单元的数据基本一致,提示其存在一个相同的骨架结构。化合物4和3的结构不同之处在于,化合物4的1H和13C -NMR数据中多一个亚甲基信号 (δH 2.26,δC 16.2)。其信号具体归属如下:1H-NMR (600 MHz,DMSO-d6):δ 5.93 (s,H-5),2.42 (m,H-7),2.26 (q,J = 7.4 Hz,H-11),1.55 (m,H-8a),1.44 (m,H-8b),1.10 (d,J = 6.9 Hz,H-10),0.94 (t,J = 7.4 Hz,H-12),0.81 (t,J = 7.4 Hz,H-9)。13C NMR (151 MHz,DMSO-d6):δ 165.8 (C-2),165.2 (C-4),164.7 (C-6),102.5 (C-3),98.8 (C-5),38.7 (C-7),26.8 (C-8),17.6 (C-10),16.2 (C-11),12.6 (C-12),11.4 (C-9)。以上数据与文献[14]基本一致,故被鉴定为germicidin A。
化合物5:棕色固体。ESI-MS显示[M+ H]+分子离子峰m/z 183.1021(calcd for C10H15O3,183.101 6),确定其相对分子质量为182,结合1H和13C-NMR数据确定其分子式为C10H14O3,不饱和度为4。该化合物氢谱存在一个双键质子δH 5.95 (1H,s) 信号,碳谱存在4个季碳信号 (δC 165.8,165.2,164.7,102.5)、1个双键次甲基碳信号 (δC 98.8),提示其含有和化合物3一样的吡喃-2-酮骨架结构。化合物5和3的分子量相同,是同分异构体,但其1H和13C -NMR数据中存在特征性的偕甲基信号 [δH 0.88 (H-9,H-10),δC 21.9 (C-9,C-10)]。具体的信号归属如下:1H-NMR (600 MHz,DMSO-d6):δ 5.92 (s,H-5),2.25 (d,J = 7.2 Hz,H-7),1.90 (m,H-8),1.72 (s,H-11),0.88 (d,J = 6.6 Hz,H-9,H-10)。13C NMR (151 MHz,DMSO-d6):δ 165.3 (C-2),165.2 (C-4),161.2 (C-6),101.1 (C-5),95.9 (C-3),41.7 (C-7),26.3 (C-8),21.9 (C-9),21.9 (C-9),8.5 (C-11)。以上数据与文献[15]基本一致,故被鉴定为germicidin I。
-
平板涂布结果显示:化合物1仅对MRSA和耻垢分支杆菌出现中等大小抑菌圈,阳性药物对相应指示菌均产生显著大小抑菌圈,其他化合物未使5种指示菌产生明显抑菌圈。
-
根据初筛结果,以MRSA和耻垢分支杆菌为指示菌,采用微量稀释法,最终确定化合物1对MRSA和耻垢分支杆菌的MIC值分别为100和64 μg/ml。
3.1. 活性初筛
3.2. 检测化合物1的MIC值
-
生物碱是一类含氮的天然药用分子,抗癌药紫杉醇、止痛药吗啡和抗疟疾的奎宁类药物均属此类[16]。文献报道,具有脂肪族侧链的三环咔唑生物碱可通过自由基清除作用保护神经细胞[9,17-18],例如lavanduquinocin,neocarazostatins和carquinostatins[7,19-20]。
课题组以一株中国南海海绵共附生链霉菌Streptomyces sp. LHW2432为研究对象,发现了2个生物碱类化合物1和2,以及3个吡喃酮类化合物3~5(图1)。化合物2此前发现于真菌和放线菌中[21-22],具有自由基清除功能[23]。有报道化合物3~5在链霉菌形态分化中具有抑制产孢的功能[24]。化合物1为三环咔唑生物碱类新天然产物,课题组发现其具有抑制MRSA和耻垢分支杆菌的微弱活性。此外,化合物1可作为化学合成药用活性三环咔唑类生物碱的重要前体[9],本研究首次发现了产生化合物1的宿主菌,为通过发酵手段廉价获取该中间体分子提供了可能。







 DownLoad:
DownLoad: