-
扶正化瘀胶囊(旧名扶正化瘀方)是针对肝纤维化“正虚血瘀”的基本病机研制而成的[1],该复方由丹参、桃仁、五味子、冬虫夏草、绞股蓝、松花粉六味药材组成[2-7],丹参在方中活血祛瘀作为君药,冬虫夏草补虚损、益精气,桃仁助丹参活血祛瘀,共为臣药,松花粉益气润燥,绞股蓝清热解毒,两者共为佐药;五味子味酸,为引经使药。从中医角度出发,丹参、冬虫夏草、桃仁、松花粉、绞股蓝、五味子这六味药材的药效物质基础主要包含脂溶性的丹参酮类和水溶性的丹酚酸类、多糖、甾醇、多种氨基酸、多种维生素、挥发油、木脂素等物质。以上六味药合用共奏活血化瘀、益精养肝之功效,适用于肝纤维化证属“瘀血阻络,肝肾不足”者[8]。其临床疗效显著,已受国家发明专利保护,获得国家中药Ⅲ类新药证书。而且2006年扶正化瘀片通过美国食品药品监督管理局审批,免于进行Ⅰ期临床,直接进入Ⅱ期临床试验,并在2013年圆满完成Ⅱ期临床实验,成为肝病领域中首个通过美国Ⅲ期临床试验的中成药[9],将来也有望成为第一个获准进入美国主流医药市场的复方中药。(标红的5处字体均用罗马字)
该复方在我国已使用多年,但由于其组分复杂,一直缺乏系统性研究,尽管目前关于该复方作用机制报道较多[10-15],然而对于整个复方的入血成分还未见报道。而且复方组成复杂,比分析单位药材的入血成分更困难,因此,明确扶正化瘀胶囊各部分的入血成分对于该复方治疗效果、作用机制的深入研究具有重要意义。
UHPLC和Q-TOF/MS的串联技术在中药复方等复杂体系研究中占有一定优势,该技术集色谱的高效分离能力和质谱的高灵敏、高分辨、强定性能力于一体,已经成为中药复方化学成分分析和鉴定的有效手段之一[16-18]。本实验采用UHPLC-QTOF/MS技术首次对扶正化瘀胶囊中的入血成分进行快速分析,并对其成分进行药材归属鉴定,进一步阐明了扶正化瘀胶囊的药效物质基础,具有重要的临床意义。
HTML
-
Agilent 1290 Infinity 液相系统,包括G4220A四元泵、G4226自动进样器G1316C柱温箱(安捷伦科技有限公司,美国);Agilent 6538 UHD and Accurate-Mass Q-TOF/MS质谱仪,配有标准电喷雾离子源(ESI)及MassHunter Qualitative Analysis Software 分析工作站(安捷伦科技有限公司,江苏);JY10001十万分之一电子天平(精密科学仪器有限公司,上海);Heal Force SMART-N 超纯水机(力康生物医疗科技控股有限公司,香港);Micro 17高速离心机(Thermo Fisher Scientific,美国);甲醇、乙腈均为色谱纯试剂(Merck,德国),甲酸为色谱级试剂(ROE scientific INC,美国),水为实验室制备的超纯水,其他试剂均为分析级。
-
丹参素、丹酚酸B、二氢丹参酮I、丹酚酸A、五味子乙素、苦杏仁苷、腺苷、五味子醇甲、绞股蓝皂苷XLIX、山奈酚对照品(一飞生物科技有限公司,纯度≥98%),批号分别为:76822-21-4、121521-90-2、87205-9-0、96574-01-5、61281-37-6。扶正化瘀胶囊(黄海制药有限责任公司,上海)购于益丰大药房,批号分别为:161220、170640、171116。
-
取丹参素、丹酚酸B、二氢丹参酮I、丹酚酸A、五味子乙素对照品适量,精密称定后,用甲醇溶解并定容成10 mg/ml的对照品储备液。吸取各对照品溶液适量,用甲醇稀释成各对照品浓度约2 mg/ml的对照品混合溶液。
-
称取扶正化瘀胶囊适量,配制成浓度为0.3 g/ml的混悬水溶液,供大鼠灌胃使用。取雄性SD大鼠6只,随机分为两组(空白组和给药组),给药前12 h禁食、不禁水,按照给药体积4 ml/kg(0.3 g/ml,临床4倍剂量)灌胃给药样品,空白组灌胃蒸馏水。给药23 min后采用眼眶取血约1.5 ml置离心管中,静置1 h后,离心(3 500 r/min,10 min)后取上清液约0.5 ml,−80 ℃冷冻保存。
-
精确吸取50 μl血清,溶于150 μl 含有内标的100%甲醇中,涡旋30 s,静置5 min,于4 ℃、1 000 r/min离心10 min,取上清液,即得血清样品。
-
色谱柱:ACQUITY UPLCHSS T3(2.1 mm×100 mm,1.8 μm,Waters Corporation,Ireland)。流动相由0.1%甲酸水溶液(A)-0.1%甲酸乙腈溶液(B)组成。梯度洗脱条件:0~3 min,2%B;3~18 min,2%~50%B;18~22 min,50%~95% B;22~25 min,95%B。平衡时间为10 min,流速为0.40 ml/min,分析所用时间为25 min。进样体积设置为3 μl,柱温箱温度为40 ℃,自动进样器的温度为4 ℃。
-
电喷雾离子源采用正、负离子模式。正离子模式条件:毛细管电压4 V;干燥气体流速11 L/min;干燥气体温度350 ℃;雾化器压力45 psig;碎片电压120 V;skimmer电压60 V。质谱采集范围从100~1 100 m/z ,参比离子为121.051和922.010 m/z。负离子模式下,除了毛细管电压为 3.5 kV,其余条件与正离子模式相同。负离子模式下的参比离子分别为 119.036 3 和 966.000 7 m/z。
-
根据国内外已有的专业数据库TCM@taiwan、TCMID(traditional Chinese medicine integrative database)和上海中科院化学专业数据库及相关研究文献,共收集了扶正化瘀胶囊六味药材中801个化学成分。利用Agilent公司研发的“Formula-Database-Generator”软件,通过各化学成分包含碳、氢、氧的个数,计算化合物精确的相对分子质量,建立化学成分的分子式和相对分子质量的数据库。
1.1. 仪器与试剂
1.2. 药品与试剂
1.3. 混合对照品溶液的制备
1.4. 血清样品的采集
1.5. 血清样品制备
1.6. 色谱条件
1.7. 质谱条件
1.8. 扶正化瘀胶囊化学成分数据库的建立
-
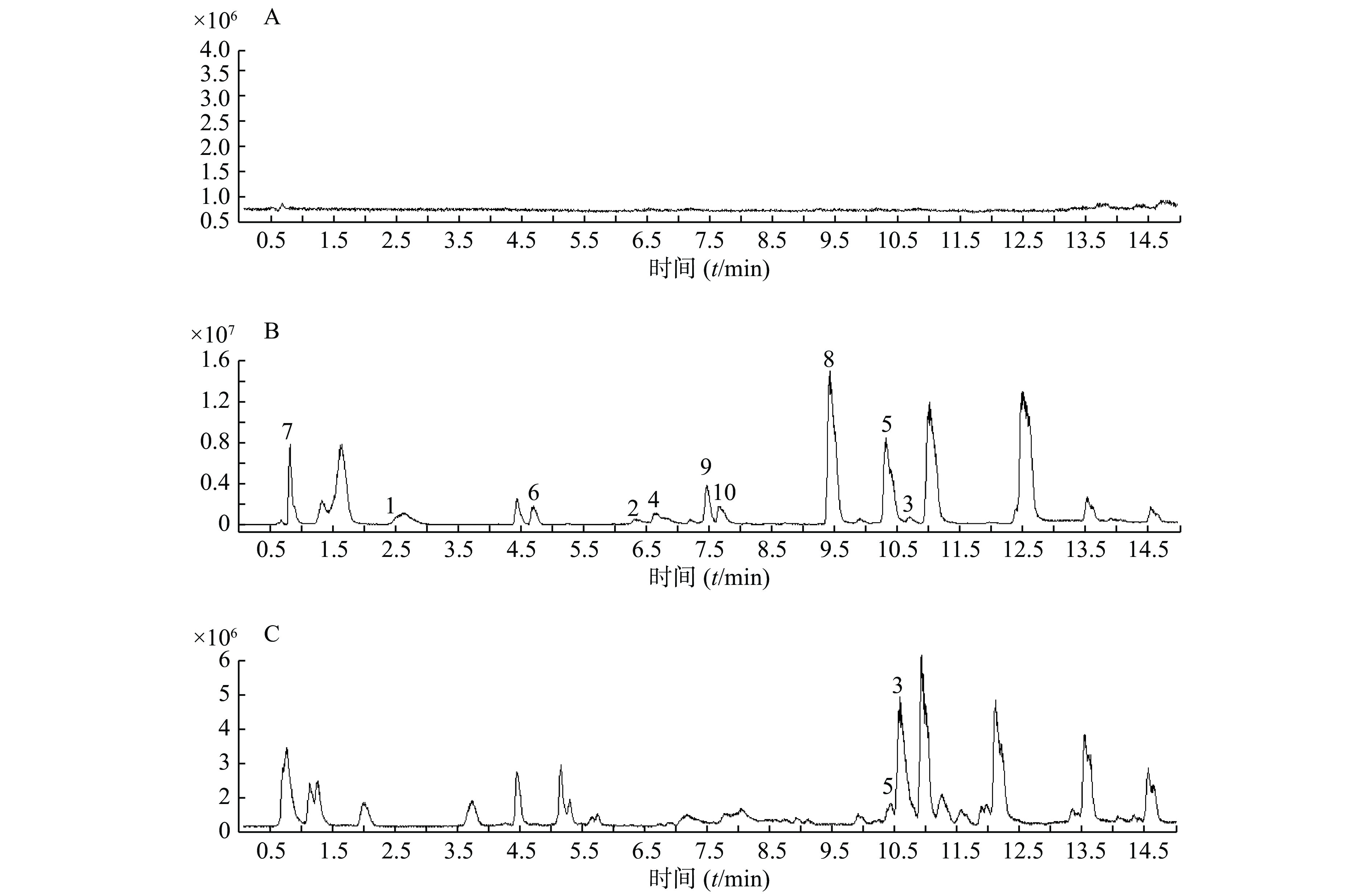
精密吸取扶正化瘀胶囊血清样品溶液和对照品混合溶液200 μl于进样小瓶,按照上述质谱和色谱测试条件进行样品分析。同时检测空白血清、混合对照品溶液和含药血清在正离子检测模式下的总离子流图,如图1所示。
-
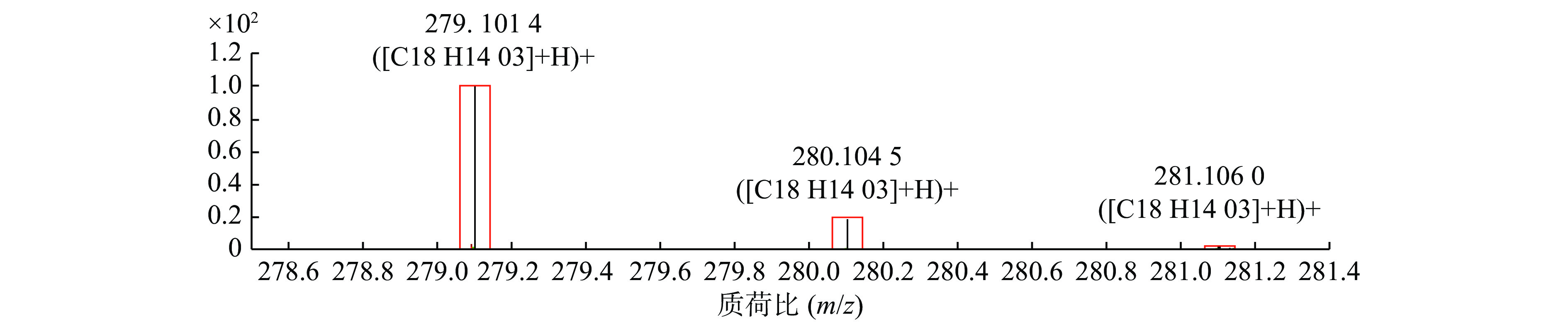
以正离子模式下3号峰二氢丹参酮I为例,说明扶正化瘀胶囊中的色谱峰鉴别过程。TIC图中的保留时间为10.453 min(图1B),色谱峰显示的准分子离子峰为279.101,利用Qualiative Analysis软件分析工具(calculator)精确计算质量数可能的元素组成(<5 ppm),结合数据库内已知的化合物质核比,可以初步确定分子式为C18H14O3。通过计算准分子离子的核素分布情况,得出同位素分布的理论值与实际值吻合良好,确定此峰为二氢丹参酮I(图2)。
-
根据飞行时间质谱测得的精确相对分子质量,比对所建的扶正化瘀胶囊化学成分数据库,应用 Qualiative Analysis质谱分析软件计算分子组成,将理论值与实测值进行比对,结合上述对照品鉴别结果及相关文献报道[19-20],对扶正化瘀胶囊血清供试品在正、负离子模式下所得的色谱图中色谱峰进一步分析,其中,血清供试品在正离子模式下初步鉴别出43个化学成分,结果见表1。在负离子模式下初步鉴别出10个化学成分,结果见表2。其中,正、负离子模式下均有响应的有4个(表中标①)。对于化学成分的药材归属,见表1。
编号 保留时间 (t/min) 化合物名称 分子式 M+X 理论分子量(m/z) 实际分子量(m/z) 误差(ppm) 归属药材 1 10.157 dihydrokaranone C15 H22 O (M+H)+ 219.174 1 219.174 3 2.01 Salvia miltiorrhiza Bge. 2 10.453 dihydrotanshinone I C18 H14 O3 (M+H)+ 279.101 0 279.101 4 0.29 Salvia miltiorrhiza Bge. 3 12.031 salvianonol C18 H20 O4 (M+H)+ 301.141 7 301.141 3 5.72 Salvia miltiorrhiza Bge. 4 10.009 isocryptotanshinone II C19 H20 O3 (M+H)+ 297.148 1 297.148 1 1.49 Salvia miltiorrhiza Bge. 5 11.078 sugiol C20 H28 O2 (M+H)+ 301.216 0 301.216 0 0.78 Salvia miltiorrhiza Bge. 6 11.636 salviol C20 H30 O2 (M+H)+ 303.231 9 303.232 2 −0.13 Salvia miltiorrhiza Bge. 7 12.762 dihydrovalepotriate C22 H32 O8 (M+H)+ 425.216 3 425.215 1 2.87 Salvia miltiorrhiza Bge. 8 7.232 4-methyl salicylaldehyde C8 H8 O2 (M+H)+ 137.059 9 137.059 8 0.05 Salvia miltiorrhiza Bge. 9 1.168 nicotinamide C6 H6 N2 O (M+H)+ 123.055 1 123.054 1 2.14 Cordyceps sinensis 10 0.642 histidine① C6 H9 N3 O2 (M+H)+ 156.075 8 156.076 8 1.52 Cordyceps sinensis 11 1.061 valine C5 H11 N O2 (M+H)+ 118.086 2 118.086 2 0.61 Cordyceps sinensis 12 1.357 adenosine C10 H13 N5 O4 (M+H)+ 268.104 2 268.104 4 −0.54 Cordyceps sinensis 13 0.765 arginine C6 H14 N4 O2 (M+H)+ 175.118 7 175.118 8 2.03 Cordyceps sinensis 14 0.634 lysine C6 H14 N2 O2 (M+H)+ 147.112 9 147.112 8 −0.45 Cordyceps sinensis 15 9.590 cis-9-octadecenoic acid C18 H34 O2 (M+NH4)+ 300.289 2 300.289 4 1.78 Cordyceps sinensis 16 9.360 octadecanoic acid C18 H36 O2 (M+NH4)+ 302.305 4 302.305 1 −0.76 Cordyceps sinensis 17 1.751 leucine C6 H13 N O2 (M+H)+ 132.102 1 132.101 3 −1.57 Cordyceps sinensis 18 1.361 pedatisectine B C5 H5 N5 (M+H)+ 136.062 2 136.061 8 1.02 Cordyceps sinensis 19 8.259 linoleic acid C18 H32 O2 (M+NH4)+ 298.274 0 298.274 1 0.9 Cordyceps sinensis 20 1.102 methionine C5 H11 N O2 S (M+H)+ 150.058 5 150.058 4 −0.61 Cordyceps sinensis 21 10.979 linoleic acid C18 H32 O2 (M+NH4)+ 298.273 9 298.274 1 2.57 Semen Persicae 22 4.726 glucose C6 H12 O6 (M+H)+ 181.070 5 181.070 8 0.95 Semen Persicae 23 9.648 GA17① C20 H26 O7 (M+Na)+ 401.158 3 401.159 2 −2.24 Semen Persicae 24 5.194 prunasin① C14 H17 N O6 (M+Na)+ 318.095 6 318.096 0 −1.94 Semen Persicae 25 9.623 gomisin R C22 H24 O7 (M+H)+ 401.158 5 401.159 1 2.71 Schisandra chinensis Fructus 26 11.168 2-(2-phenyl cyclohexyloxy) ethanol C14 H20 O2 (M+Na)+ 243.135 3 243.135 8 1.78 Schisandra chinensis Fructus 27 9.911 deangeloylgomisin F C23 H28 O8 (M+Na)+ 455.168 9 455.168 2 −0.19 Schisandra chinensis Fructus 28 12.524 schisandrin B C23 H28 O6 (M+Na)+ 423.177 5 423.177 6 1.88 Schisandra chinensis Fructus 29 8.333 gomisin Q C24 H32 O8 (M+Na)+ 471.198 1 471.198 2 1.56 Schisandra chinensis Fructus 30 9.130 (±)-gomisin m1 C22 H26 O6 (M+Na)+ 409.161 8 409.161 8 0.64 Schisandra chinensis Fructus 31 11.973 1,1alpha,4,5,6,7,7alpha,7beta-octahydro-1,1,7,7alpha-tetramethyl-2h-cyclopropa (alpha)-naphthalen-2-one C14 H22 O (M+H)+ 207.174 9 207.174 2 −2.61 Schisandra chinensis Fructus 32 6.870 1-phenyl-1,3-butanedion C10 H10 O2 (M+H)+ 163.075 5 163.075 8 −1.40 Schisandra chinensis Fructus 33 10.839 geranyl acetate C12 H20 O2 (M+H)+ 197.153 8 197.153 5 1.07 Schisandra chinensis Fructus 34 11.110 tigloylgomisin P C28 H34 O9 (M+NH4)+ 532.254 3 532.253 8 0.22 Schisandra chinensis Fructus 35 7.322 citronellyl acetate C12 H22 O2 (M+NH4)+ 216.195 3 216.195 4 2.48 Schisandra chinensis Fructus 36 0.814 gama-octalactone C8 H14 O2 (M+NH4)+ 160.133 0 160.132 9 0.27 Schisandra chinensis Fructus 37 9.607 gomisin D C28 H34 O10 (M+Na)+ 553.204 2 553.203 9 0.61 Schisandra chinensis Fructus 38 10.248 1,1α,2,4,6,7,7α,7β-octahydro-1,1,7,7α-tetra-methyl-5h-cyclopropa (α)-naphthalen-5-one C15 H22 O (M+H)+ 219.173 7 219.174 3 2.92 Schisandra chinensis Fructus 39 9.935 phenyl-2-propanone C9 H10 O (M+H)+ 135.080 2 135.080 2 1.81 Schisandra chinensis Fructus 40 11.834 prehispanolone① C20 H30 O3 (M+H)+ 319.226 3 319.225 7 2.56 Schisandra chinensis Fructus 41 9.919 psilostachyin C15 H20 O5 (M+H)+ 281.137 9 281.138 4 1.67 Schisandra chinensis Fructus 42 11.324 santlic acid C15 H22 O2 (M+H)+ 235.168 3 235.170 5 0.58 Schisandra chinensis Fructus 43 7.355 gomisin A C23 H28 O7 (M+H)+ 417.190 6 417.190 3 0.23 Schisandra chinensis Fructus 注:“①”表示正、负离子模式下都已鉴别出。 编号 保留时间 (t/min) 化合物名称 分子式 M-X 理论分子量(m/z) 实际分子量(m/z) 误差(ppm) 归属药材 1 6.795 tanshindiol C C18 H16 O5 (M+COOH)− 357.098 9 357.099 7 −1.34 Salvia miltiorrhiza Bge. 2 13.681 paramiltioic acid C19 H24 O5 (M+COOH)− 377.161 6 377.161 9 −1.84 Salvia miltiorrhiza Bge. 3 0.812 histidine① C6 H9 N3 O2 (M-H)− 154.062 6 154.061 2 −2.28 Cordyceps sinensis 4 0.764 glutamic acid C5 H9 N O4 (M-H)− 146.045 7 146.044 9 1.02 Gynostemma pentaphyllum (Thunb.) Makino 5 13.632 GA17① C20 H26 O7 (M-H)− 377.161 6 377.161 9 −2.18 Semen Persicae 6 3.714 GA19 C20 H26 O6 (M+COOH)− 407.171 9 407.171 7 0.02 Semen Persicae 7 13.632 GA119 C19 H24 O5 (M+COOH)− 377.161 6 377.161 9 −2.48 Semen Persicae 8 5.209 prunasin① C14 H17 N O6 (M+COOH)− 340.102 9 340.102 3 0.40 Semen Persicae 9 11.060 prehispanolone① C20 H30 O3 (M-H)− 317.211 5 317.210 9 2.19 Schisandra chinensis Fructus 10 14.158 schisandrone C21 H24 O5 (M-H)− 355.156 4 355.156 6 −2.79 Schisandra chinensis Fructus 注:“①”表示正、负离子模式下都已鉴别出。
2.1. 血清样品总离子流图
2.2. 利用对照品鉴别化合物
2.3. 化合物鉴别
2.4. 扶正化瘀胶囊中入血成分的鉴别结果
-
本研究首次运用UHPLC-Q-TOF/MS对扶正化瘀胶囊入血成分进行分析,该方法效率高、稳定性好、灵敏度高,能快速检测出含量较低的化学成分,优于其他方法。利用该技术快速初步鉴别出扶正化瘀胶囊血清供试品共49个化学成分,推测其可能为扶正化瘀胶囊发挥药效的物质基础。已鉴别出的化学成分主要集中在丹参、冬虫夏草,符合中药复方“君臣佐使”的配伍原则。为进一步探究其作用机制,拟进一步从 49 种入血成分筛选出抗肝纤维化的体内、外活性测试,期望筛选出具有抗肝纤维化的活性单体。该研究进一步将相应物质的色谱峰明确化,为扶正化瘀胶囊的深入研究奠定了良好基础。








 DownLoad:
DownLoad: