-
由于免疫缺陷人群的增加和新真菌物种的出现,全球人口中侵袭性真菌感染(IFIs)越来越频繁[1-2]。IFIs对人类的发病率和死亡率有重大影响,全球每年造成约150万人死亡,并且这个数字还在增加[3-4]。
有研究发现,重症新型冠状病毒肺炎患者患侵袭性真菌感染的风险增加,有患者的临床相关标本中发现真菌标记物[5-6]。与非新冠肺炎患者相比,新冠肺炎患者中的侵袭性真菌感染发病率和死亡率都更高[7]。
目前临床常用的抗真菌药物有多烯类、唑类、棘白菌素和氟胞嘧啶等4类,其中氮唑类药物作为治疗和预防侵袭性真菌感染的一线疗法[8-9]。然而现有抗真菌药物的耐药性是一个日益严重的问题,具有可变敏感性或获得性耐药性的真菌种类数量呈增长趋势[3],且交叉耐药已被广泛报道[10]。有研究发现感染具有氟康唑和伏立康唑耐药念珠菌分离株患者的临床预后明显较差[11]。因此,开发具有高敏感的新型抗真菌药物迫在眉睫。
-
MSL300型核磁共振仪(CDCl3为溶剂,TMS为内标,Bruker公司);1260-1620LC-MS液相质谱联用仪、UPLC-QTOF/MS高分辨质谱仪(Agilent公司);HB10S096型旋转蒸发仪(IKA公司);2537型紫外分析仪(上海科艺光学仪器厂);DLSB型低温冷却液循环泵、B20-8-YK型耐腐蚀隔膜泵(上海豫康科教仪器设备有限公司)。监测反应使用的薄层色谱硅胶板(以硅胶GF254为固定相)和柱层析所用硅胶由烟台江友硅胶开发有限公司提供。起始原料(化合物1)、各类试剂均为市售分析纯或化学纯。
-
三唑类抗真菌药的作用机制是通过抑制对真菌细胞色素P450具有依赖性的羊毛甾醇14α-去甲基化酶,进而导致真菌细胞膜上麦角甾醇缺失,破坏真菌细胞立体结构的完整性,最终导致真菌的死亡[3,12]。根据国内外同行对三氮唑类化合物的研究,我们发现1,2,3-三唑是一种医学上有特殊意义的结构,在许多生物活性分子和药物中作为关键的结构特征。许多含有1,2,3-三唑结构的分子具有抗结核、抗真菌、抗过敏、抗病毒、抗肿瘤、抗疟疾作用以及神经保护活性[8,13-16]。
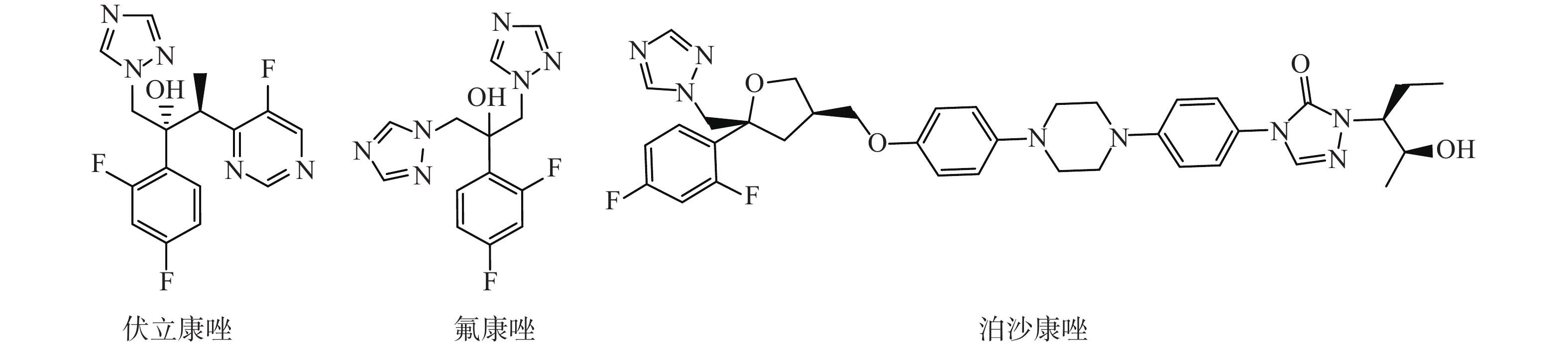
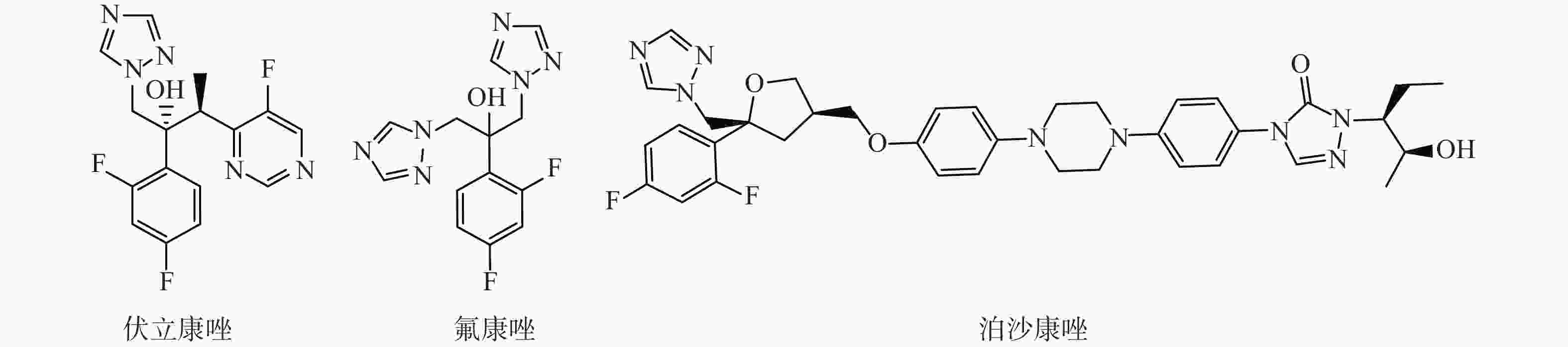
根据前人对氮唑类药物所作的构效关系的研究,其活性必需基团为叔醇结构和三唑环,2,4-二氟苯基为重要药效基团[17]。在此基础上,我们开展了以氟康唑为先导药物,设计合成新衍生物,引入不同的胺基侧链,尔后引入三氮唑环、各种取代苄基,观察活性情况。目标是获得高活性抗耐药的新型氮唑类化合物,丰富三氮唑类化合物的结构类型和构效关系。
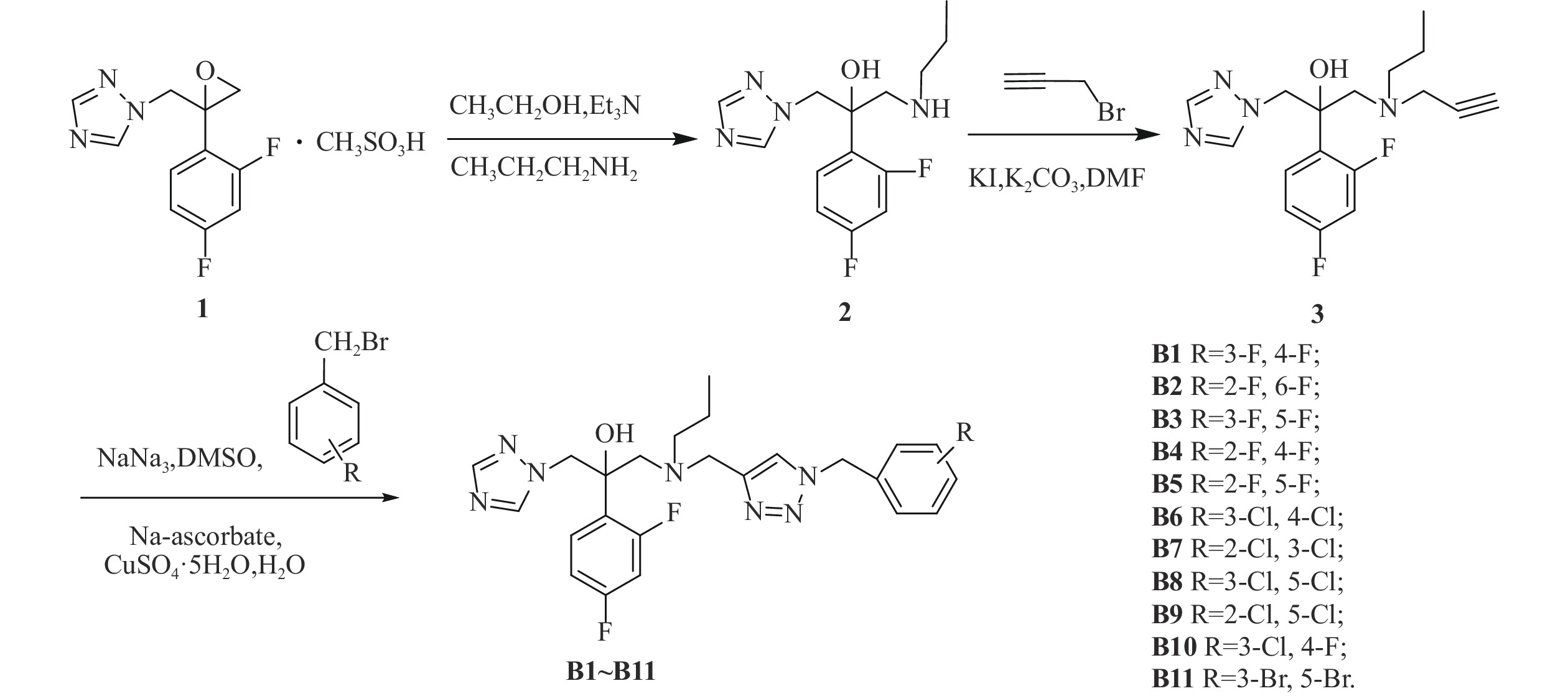
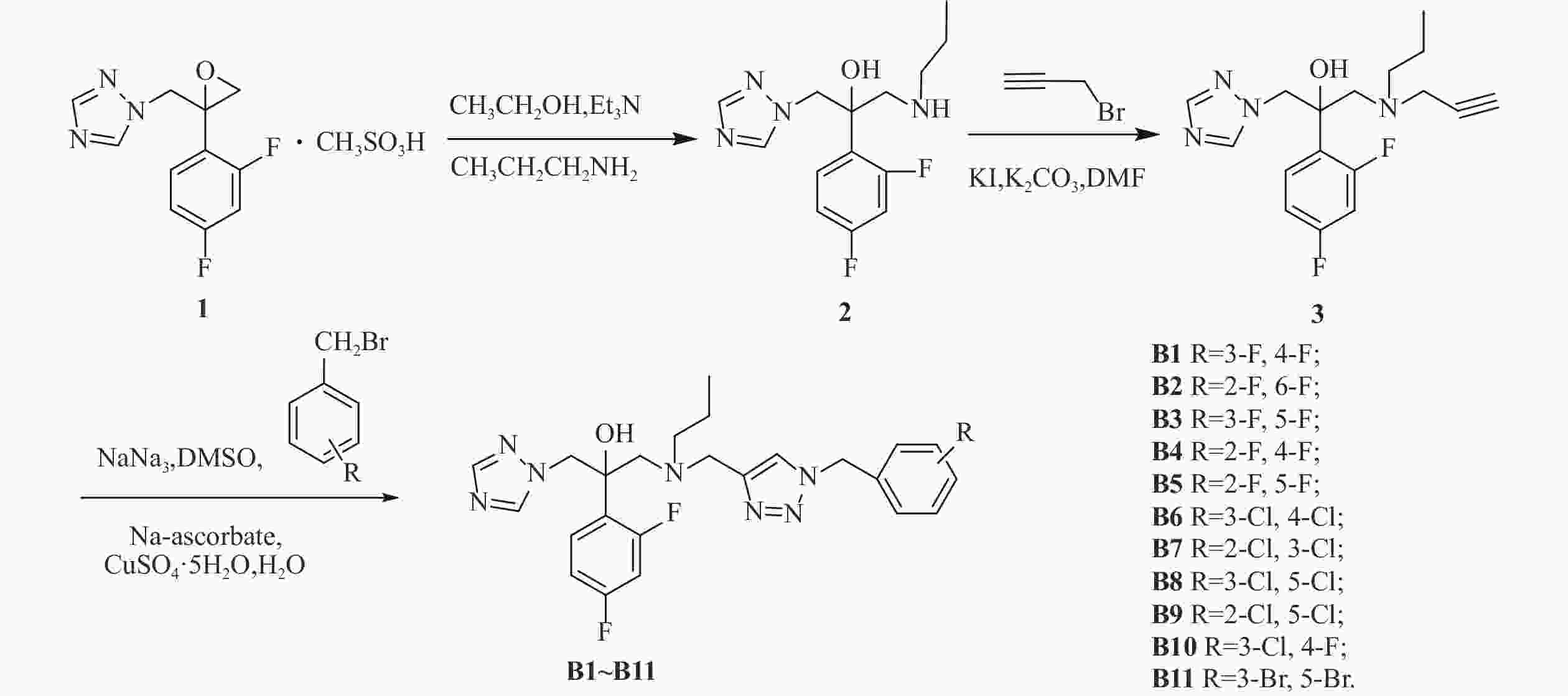
我们以1-[2-(2,4-二氟苯基)-2,3环氧丙基]-1H-1,2,4-三唑甲磺酸盐为起始原料,设计和合成了一系列氟康唑的类似物,结构为1-(1H-1,2,4-三唑-1-基)-2-(2,4-二氟苯基)-3-[N-正丙基-N-((1-取代-1H-1,2,3-三唑-4基)甲基)氨基]-2-丙醇类化合物。通过对3种人体致病真菌的初步抑菌试验,显示部分化合物具有一定的抗真菌活性。
目标化合物的合成路线如图1所示。
-
在500 ml的圆底烧瓶中加入化合物1(21 g, 63.05 mmol),正丙胺10 ml, 三乙胺20 ml,于250 ml乙醇中加热回流6~8 h[18],TLC监测,反应完毕后蒸除溶剂,用200 ml乙酸乙酯萃取,100 ml水洗2次,无水硫酸钠静置、干燥。过滤后旋蒸除掉乙酸乙酯,得油状的化合物2(12.042 g, 40.66 mmol),收率64.49%。
-
在250 ml的茄形瓶中加入化合物2(12.042 g, 40.66 mmol),溴丙炔(6.965 g, 58.55 mmol),无水碳酸钾(8.092 g, 58.55 mmol),碘化钾(675 mg, 4.066 mmol),于50 ml N,N-二甲基甲酰胺中室温下反应8 h[19],TLC监测,反应完毕后,用50 ml乙酸乙酯提取,100 ml水洗2次,无水硫酸钠静置、干燥。过滤后旋蒸除掉乙酸乙酯,柱层析[流动相为石油醚/乙酸乙酯(V/V)=1/1]得化合物3,收率76.27%。
-
在50 ml茄形瓶中加入叠氮钠(164 mg, 2.52 mmol)、3,4-二氟溴苄(261 mg, 1.26 mmol),二甲亚砜10 ml,室温下磁力搅拌反应3~5 h,然后加入化合物3(210 mg, 0.63 mmol),抗坏血酸钠 (20 mg, 0.10 mmol),无水硫酸铜 (25 mg, 0.10 mmol),水1 ml,室温搅拌反应10 min[19],TCL监测至化合物3反应完毕。将反应液倒入稀氨水中,乙酸乙酯萃取(30 ml×2次),乙酸乙酯层再用稀盐酸酸化萃取(30 ml×2次),分出水层,水层加无水碳酸钠调pH至7左右,乙酸乙酯萃取(30 ml×2次),乙酸乙酯层用无水硫酸钠干燥4 h后,过滤,减压抽溶剂得目标产物,收率67.99%。
-
方法同化合物B1,收率50.10%~95.83%。
中间体及目标化合物的波谱数据见表1, 部分化合物的高分辨质谱数据见表2。
化合物 R 波谱数据 3 1H NMR(300 MHz, CDCl3) δ 8.14 (1H, s, triazole-H), 7.80 (1H, s, triazole-H), 7.59-7.50 (1H, q, Ar-H), 6.85-6.78 (2H, m, Ar-H), 5.06 (1H, s, -OH), 4.60-4.48 (2H, q, J = 11.35 Hz, –CH2), 3.32-3.27 (1H, dd, J=13.95 Hz, –CH2), 3.19-3.02 (2H, m, –CH2), 2.76-2.72 (1H, d, J = 13.96 Hz, –CH), 2.46-2.37 (1H, m, –CH2), 2.33-2.24 (1H, m, –CH2), 2.15 (1H, t, J=2.22 Hz, –CH), 1.34-1.22 (2H, m, –CH2), 0.79-0.74 (3H, t, J=7.35 Hz, –CH3).
13C NMR(75 MHz, CDCl3) δ 164.51-161.04, 160.69-157.27, 151.09, 144.68, 129.52, 125.97, 111.49, 104.30, 78.12, 73.06, 72.37, 58.43, 57.03, 56.19, 43.37, 20.61, 11.40.B1 3-F, 4-F 1H NMR(300 MHz, CDCl3) δ 8.10 (1H, s, triazole-H), 7.77 (1H, s, triazole-H), 7.59 (1H, q, Ar-H), 7.18 (1H, d, triazole-H), 7.05 (3H, d, Ar-H), 6.80 (2H, m, Ar-H), 5.49 (2H, s, –CH2) , 4.56-4.51 (1H, d, J=13.80 Hz, –CH2), 4.01-4.36 (1H, d, J=13.71 Hz, –CH2), 3.65-3.49 (2H, q, J=16.28 Hz, –CH2), 3.19-3.14 (1H, d, J=13.24 Hz, –CH2), 2.73-2.68 (1H, d, J=13.90 Hz, –CH2), 2.27-2.17 (2H, q, J=29.82 Hz, –CH2), 1.29 (2H, s, –CH2), 0.70-0.65 (3H, t, J=6.78 Hz, –CH3). B2 2-F, 6-F 1H NMR(300 MHz, CDCl3) δ 8.10 (1H, d, triazole-H), 7.75 (1H, s, triazole-H), 7.60-7.53 (1H, q, Ar-H), 7.42-7.32 (1H, m, Ar-H), 7.20 (1H, s, triazole-H), 7.00-6.95 (2H, t, Ar-H), 6.82-6.73 (2H, m, Ar-H), 5.61 (2H, s, –CH2), 4.51-4.38 (2H, q, J=13.55 Hz, –CH2), 3.61-3.46 (2H, q, J=14.43 Hz, –CH2), 3.17-3.13 (1H, d, J=14.20 Hz, –CH2), 2.73-2.69 (1H, d, J=13.63 Hz, –CH2), 2.24 (2H, s, –CH2), 1.27 (2H, s, –CH2), 0.70-0.65 (3H, t, J=6.98 Hz, –CH3). B3 3-F, 5-F 1H NMR(300 MHz, CDCl3) δ 8.08 (1H, d, triazole-H), 7.76 (1H, s, triazole-H), 7.63-7.55 (1H, m, Ar-H), 7.11 (1H, s, triazole-H), 6.85-6.74 (5H, m, Ar-H), 5.52 (2H, s, –CH2), 4.55-4.51 (1H, d, J=14.25 Hz, –CH2), 4.41-4.36 (1H, d, J=14.25 Hz, –CH2), 3.66-3.50 (2H, q, J=16.61 Hz, –CH2), 3.19-3.14 (1H, d, J=14.06 Hz, –CH2), 2.73-2.68 (1H, d, J=14.00 Hz, –CH2), 2.34-2.25 (1H, m, –CH2), 2.21-2.11 (1H, m, –CH2), 1.36-1.25 (2H, m, –CH2), 0.70-0.65 (3H, t, J=7.34 Hz, –CH3). B4 2-F, 4-F 1H NMR(300 MHz, CDCl3) δ 8.09 (1H, d, triazole-H), 7.76 (1H, s, triazole-H), 7.63-7.54 (1H, m, Ar-H), 7.31 (1H, t, Ar-H), 7.17 (1H, s, triazole-H), 6.91-6.85 (2H, t, Ar-H), 6.84-6.74 (2H, t, Ar-H), 5.53 (2H, s, –CH2), 4.54-4.37 (2H, q, J=17.08 Hz, –CH2), 3.58-3.53 (2H, d, J=15.43 Hz, –CH2), 3.18-3.14 (1H, d, J=11.92 Hz, –CH2), 2.74-2.71 (1H, d, J=9.30 Hz, –CH2), 2.27-2.20 (2H, d, J=20.71 Hz, –CH2), 1.29-1.25 (2H, d, J=12.28 Hz, –CH2), 0.70-0.66 (3H, t, J=6.27 Hz, –CH3). B5 2-F, 5-F 1H NMR(300 MHz, CDCl3) δ 8.10 (1H, d, triazole-H), 7.77 (1H, s, triazole-H), 7.63-7.55 (1H, m, Ar-H), 7.20 (1H, s, triazole-H), 7.11-7.05 (2H, m, Ar-H), 6.94 (1H, s, Ar-H), 6.84-6.75 (2H, m, Ar-H), 5.56 (2H, s, –CH2), 4.55-4.39 (2H, q, J=16.18 Hz, –CH2), 3.64-3.50 (2H, d, J=13.86 Hz, –CH2), 3.20-3.15 (1H, d, J=15.01 Hz, –CH2), 2.75-2.71 (1H, d, J=13.51 Hz, –CH2), 2.29-2.22 (2H, d, J=21.24 Hz, –CH2), 1.30 (2H, s, –CH2), 0.69 (3H, s, –CH3). B6 3-Cl, 4-Cl 1H NMR(300 MHz, CDCl3) δ 8.11 (1H, d, triazole-H), 7.78 (1H, s, triazole-H), 7.63-7.55 (1H, m, Ar-H), 7.47-7.44 (1H, d, Ar-H), 7.35 (1H, s, triazole-H), 7.11-7.09 (2H, d, Ar-H), 6.84-6.75 (2H, m, Ar-H), 5.49 (2H, s, –CH2), 4.56-4.52 (1H, d, J=14.13 Hz, –CH2), 4.41-4.36 (1H, d, J=14.25 Hz, –CH2), 3.66-3.49 (2H, q, J=16.49 Hz, –CH2), 3.19-3.14 (1H, d, J=13.33 Hz, –CH2), 2.73-2.68 (1H, d, J=13.27 Hz, –CH2), 2.28-2.17 (2H, d, J=31.32 Hz, –CH2), 1.29 (2H, s, –CH2), 0.70-0.65 (3H, t, J=7.24 Hz, –CH3). B7 2-Cl, 3-Cl 1H NMR(300 MHz, CDCl3) δ 8.13 (1H, d, triazole-H), 7.77 (1H, s, triazole-H), 7.62-7.54 (1H, m, Ar-H), 7.50-7.47 (1H, dd, Ar-H), 7.25-7.19 (2H, t, Ar-H), 7.05 (1H, d, triazole-H), 6.84-6.73 (2H, m, Ar-H), 5.68 (2H, s, –CH2), 4.55-4.39 (2H, q, J=15.71 Hz, –CH2), 3.64-3.50 (2H, q, J=14.53 Hz, –CH2), 3.19-3.14 (1H, d, J=14.05 Hz, –CH2), 2.75-2.70 (1H, d, J=13.48 Hz, –CH2), 2.28-2.22 (2H, d, J=20.34 Hz, –CH2), 1.29 (2H, s, –CH2), 0.71-0.65 (3H, t, J=7.28 Hz, –CH3). B8 3-Cl, 5-Cl 1H NMR(300 MHz, CDCl3) δ 8.12 (1H, d, triazole-H), 7.78 (1H, s, triazole-H), 7.64-7.56 (1H, m, Ar-H), 7.36 (1H, s, triazole-H), 7.13 (3H, s, Ar-H), 6.86-6.75 (2H, m, Ar-H), 5.49 (2H, s, –CH2), 4.58-4.53 (1H, q, J=13.92 Hz, –CH2), 4.42-4.37 (1H, q, J=14.61 Hz, –CH2), 3.62-3.55 (2H, d, J=21.03 Hz, –CH2), 3.20-3.15 (1H, d, J=13.23 Hz, –CH2), 2.72-2.68 (1H, d, J=10.65 Hz, –CH2), 2.29-2.18 (2H, d, J=35.37 Hz, –CH2), 1.30 (2H, s, –CH2), 0.69 (3H, s, –CH3). B9 2-Cl, 5-Cl 1H NMR(300 MHz, CDCl3) δ 8.10 (1H, d, triazole-H), 7.76 (1H, s, triazole-H), 7.63-7.54 (1H, m, Ar-H), 7.39-7.31 (2H, t, Ar-H), 7.21 (1H, s, triazole-H), 7.13 (1H, s, Ar-H), 6.84-6.75 (2H, m, Ar-H), 5.61 (2H, s, –CH2), 4.55-4.50 (1H, d, J=13.47 Hz, –CH2), 4.44-4.39 (1H, q, J=14.43 Hz, –CH2), 3.65-3.51 (2H, q, J=14.43 Hz, –CH2), 3.20-3.16 (1H, d, J=13.14 Hz, –CH2), 2.75-2.70 (1H, d, J=13.55 Hz, –CH2), 2.29-2.22 (2H, d, J=21.33 Hz, –CH2), 1.28 (2H, s, –CH2), 0.69 (3H, s, –CH3). B10 3-Cl, 4-F 1H NMR(300 MHz, CDCl3) δ 8.13 (1H, d, triazole-H), 7.78 (1H, s, triazole-H), 7.62-7.54 (1H, m, Ar-H), 7.32-7.30 (1H, d, Ar-H), 7.15-7.13 (2H, d, Ar-H), 7.09 (1H, s, triazole-H), 6.83-6.73 (2H, m, Ar-H), 5.47 (2H, s, –CH2), 4.55-4.36 (2H, q, J=19.44 Hz, –CH2), 3.65-3.48 (2H, q, J=16.33 Hz, –CH2), 3.18-3.13 (1H, d, J=13.91 Hz, –CH2), 2.72-2.68 (1H, d, J=13.77 Hz, –CH2), 2.33-2.24 (1H, m, –CH2), 2.20-2.10 (1H, m, –CH2), 1.29 (2H, s, –CH2), 0.69-0.64 (3H, t, J=7.31 Hz, –CH3).
13C NMR(75 MHz, CDCl3) δ 167.88-164.42, 164.02-160.59, 163.30, 159.98, 154.85, 148.29, 135.22, 133.72, 133.15, 131.26, 129.69, 125.38, 125.14, 120.71, 114.88, 107.58, 75.57, 61.42, 60.72, 59.63, 56.25, 52.75, 23.57, 14.81.B11 3-Br, 5-Br 1H NMR(300 MHz, CDCl3) δ 8.08 (1H, d, triazole-H), 7.77 (1H, s, triazole-H), 7.66 (1H, s, Ar-H), 7.63-7.55 (1H, m, Ar-H), 7.32 (2H, d, Ar-H), 7.10 (1H, d, triazole-H), 6.85-6.74 (2H, m, Ar-H), 5.47 (2H, s, –CH2), 5.33 (1H, s, -OH), 4.56-4.52 (1H, d, J=14.16 Hz, –CH2), 4.41-4.36 (1H, d, J=14.22 Hz, –CH2), 3.67-3.50 (2H, q, J=16.40 Hz, –CH2), 3.20-3.16 (1H, d, J=13.56 Hz, –CH2), 2.73-2.68 (1H, d, J=14.27 Hz, –CH2), 2.29-2.19 (2H, d, J=29.70 Hz, –CH2), 1.29 (2H, s, –CH2), 0.71-0.66 (3H, t, J=7.31 Hz, –CH3).
13C NMR(300 MHz, CDCl3) δ 164.50-161.03, 160.61-157.19, 150.88, 144.93, 138.40, 134.51, 129.75, 129.61, 129.61, 127.97, 126.35, 123.64, 123.64, 122.10, 111.51, 104.18, 72.23, 58.02, 57.36, 56.07, 52.66, 49.39, 20.18, 11.40.化合物 R ChemDraw提示分子量 HRMS测得分子量 3 334.16 335.1694 B2 2-F, 6-F 503.21 504.2127 B3 3-F, 5-F 503.21 504.2141 B5 2-F, 5-F 503.21 504.2152 B7 2-Cl, 3-Cl 535.15 536.1546 -
选取了3种实验真菌,菌株由海军军医大学药学系军特药研究中心提供,阳性对照药为伏立康唑(VCZ)、泊沙康唑(POS)和氟康唑(FCZ),对照药结构式见图2。
测试化合物体外抑菌活性的实验采用了美国国家临床实验室标准委员会(NCCLS)提出的标准化抗真菌敏感性实验方法。目标化合物对3种致病菌(C.alb SC5314, C.neo, A.fum.)的体外抑菌活性测试结果如表3所示。
化合物 R C.alb SC5314 C.neo h99 A.fum 7544 B1 3-F, 4-F 2 16 >64 B2 2-F, 6-F 1 8 >64 B3 3-F, 5-F 4 8 >64 B4 2-F, 4-F 1 4 >64 B5 2-F, 5-F 2 8 >64 B6 3-Cl, 4-Cl 2 8 >64 B7 2-Cl, 3-Cl 1 8 >64 B8 3-Cl, 5-Cl 4 >64 >64 B9 2-Cl, 5-Cl 1 16 >64 B10 3-Cl, 4-F 1 1 >64 B11 3-Br, 5-Br 0.125 2 >64 VCZ 0.0156 0.125 0.125 POS 0.125 0.5 1 FCZ 0.25 8 >64 注 :C.alb: 白念珠菌;C.neo: 新型隐球菌;A.fum: 烟曲霉菌;VCZ: 伏立康唑; POS: 泊沙康唑;FCZ: 氟康唑。 -
根据目标化合物的体外抗真菌活性结果,化合物B11对白念珠菌SC5314的活性与泊沙康唑相当,是氟康唑的2倍; 化合物B2、B3、B5、B6、B7对新型隐球菌H99的活性与氟康唑相当,化合物B10、B11、B4对新型隐球菌h99的活性是氟康唑的2~4倍;所有化合物对烟曲霉菌活性欠佳。总体来看,活性最好的3个化合物依次是化合物B11、B10、B4,R取代基分别为3-Br, 5-Br、3-Cl, 4-F、2-F、4-F,侧链中均含有正丙基,对除烟曲霉菌以外的其他2种真菌的抑制活性比较突出,分析可能是正丙基作为疏水基团与靶酶的疏水腔结合较好,苯基上间位的取代基减小了分子空间位阻,提高了化合物的抗真菌活性。由于本实验合成的化合物数量有限,更深入的构效关系探讨有待于进一步的研究。
Design, synthesis and antifungal activity of novel triazoles containing propyl side chains
doi: 10.12206/j.issn.2097-2024.202208016
- Received Date: 2022-08-03
- Rev Recd Date: 2022-11-01
- Available Online: 2023-07-14
- Publish Date: 2023-02-25
-
Key words:
- triazole derivatives /
- N-propyl side chain /
- disubstituted benzyl group /
- synthesis /
- antifungal activity
Abstract:
| Citation: | ZHANG Haidong, YUAN Fumiao, ZHANG Dazhi, JIANG Yuanying, YU Shichong. Design, synthesis and antifungal activity of novel triazoles containing propyl side chains[J]. Journal of Pharmaceutical Practice and Service, 2023, 41(2): 86-90. doi: 10.12206/j.issn.2097-2024.202208016 |







 DownLoad:
DownLoad: