-
放线菌以其能产生结构新颖且有良好生物活性的先导化合物而备受关注[1],一直被认为是天然药物的重要生产者,其主要结构类型包括聚酮、生物碱、多肽和萜烯类化合物等,同时涵盖了多种多样的生物活性如抗菌、抗寄生虫、免疫调节、抗炎、抗癌等[2-4],这突显了放线菌具有不可预估的药物开发潜力。
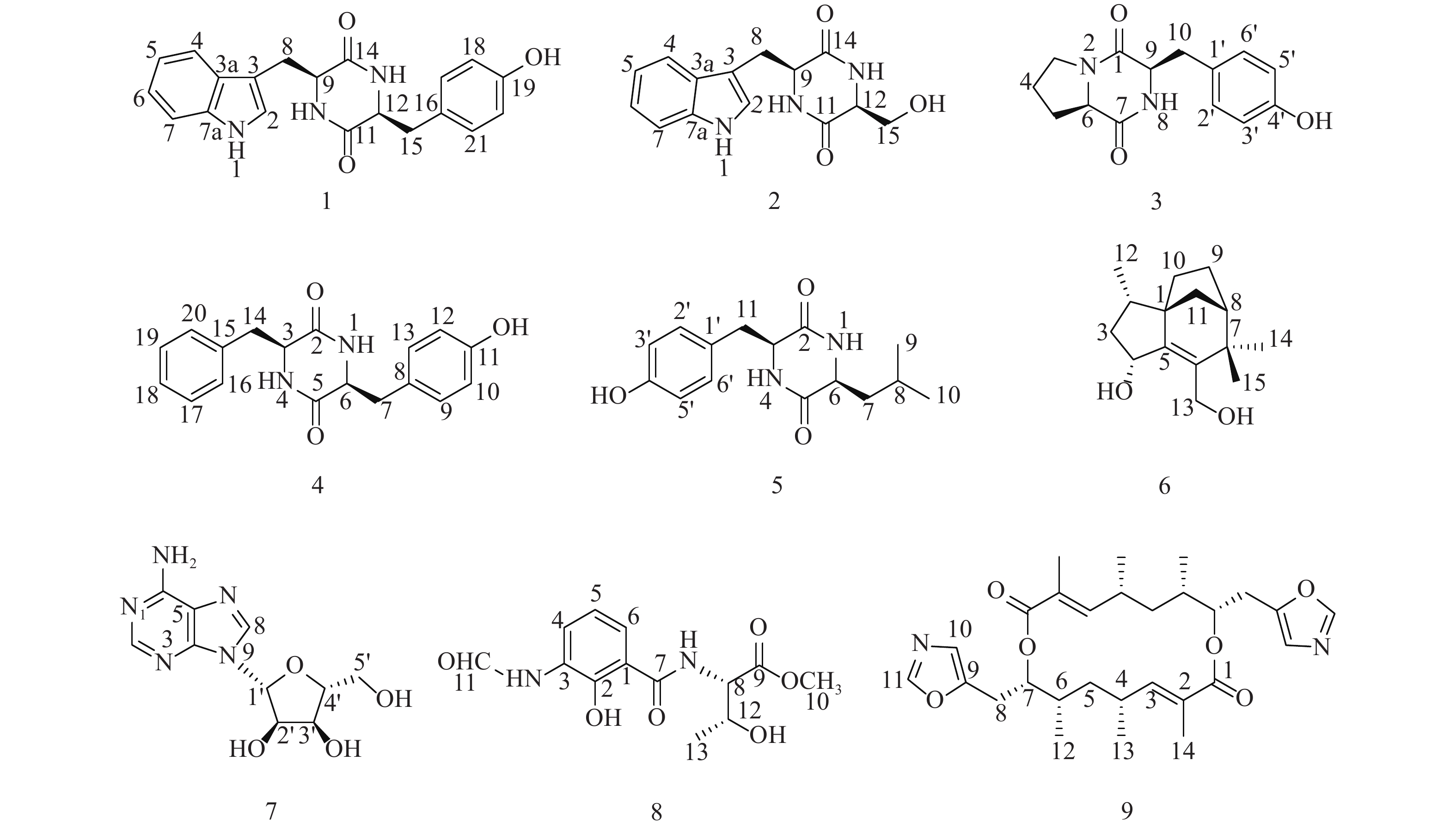
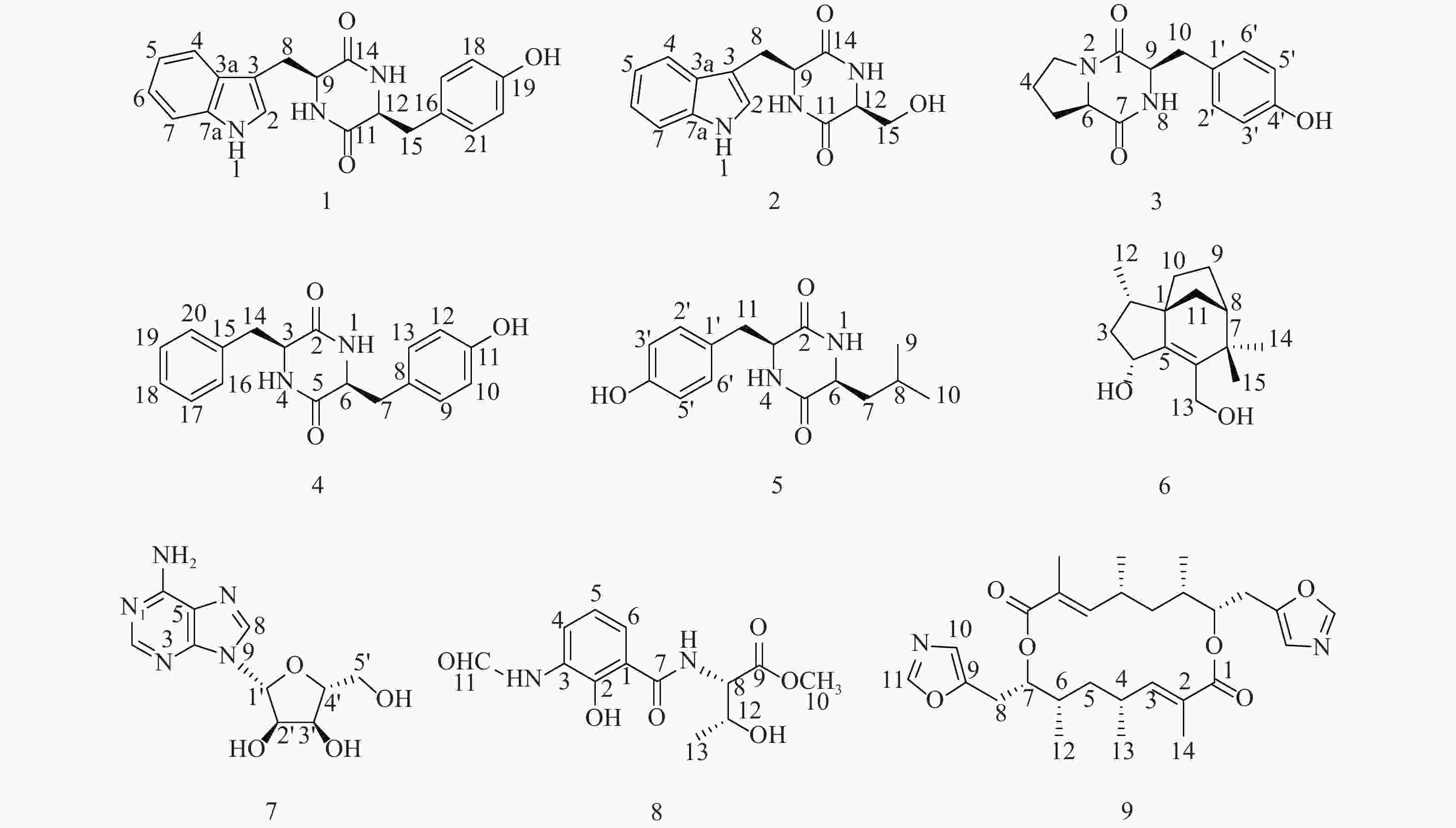
随着研究的深入,陆地和普通环境中的资源日趋枯竭,很多微生物及其次生代谢产物被重复开发和提取分离,发现新活性分子的几率愈来愈低,开发创新药物的难度越来越大[5-6];而极地极端的生态环境造就的微生物具有产生更为特别的化学骨架和活性次生代谢产物的能力,是新型药源分子的重要来源。本文以采自北极楚克奇海域海绵共附生放线菌Streptomyces sp. LHW11-07为研究对象,从其发酵浸膏中分离鉴定了9个单体化合物1~9(图1),其中化合物1和2为该属内首次分离得到。
-
AMX-600型核磁共振仪(德国Bruker公司);Xevo G2-XS Q-TOF液质联用仪、1525/2996, 2998型高效液相色谱仪(美国Waters公司);半制备型HPLC色谱柱(Atlantis Prep T3,美国Waters公司;YMC C18,日本YMC公司);中压柱色谱仪(法国Interchim公司);恒温振荡培养箱(上海一恒科学仪器有限公司);N-1000型旋转蒸发仪(上海爱郎仪器有限公司);反相ODS硅胶和Sephadex LH-20柱色谱填料(Pharmacia公司);正相硅胶(200-300目)和TLC薄层板(烟台江友硅胶开发有限公司);分析级试剂(上海化学试剂公司);色谱级试剂(德国Merck公司);氘代试剂(美国剑桥同位素实验室公司)。
-
菌株分离于北极海域来源的海绵样本,经16S rRNA基因序列鉴定为Streptomyces sp.,编号为LHW11-07,菌种保存于上海交通大学医学院附属仁济医院药学部海洋药物研究中心。
-
培养基为ISP2:葡萄糖(4 g/L)、酵母提取物(4 g/L)、麦芽糖提取物(10 g/L)以及海盐(25 g/L),加水溶解后调节pH为7.2~7.4,分装后高压灭菌20 min (121 ℃),冷却备用。
挑取Streptomyces sp. LHW11-07单菌落至1级种子培养基里(100 ml ISP2培养基至250 ml三角瓶),置于30 ℃,220 r/min的恒温摇床培养3 d,得1级种子液;将1级种子液按5%接种量接到2级种子培养基里(150 ml ISP2培养基至500 ml三角瓶),置于30 ℃,220 r/min的恒温摇床培养3 d,得2级种子液;将2级种子液按5%接种量接到大发酵培养基里(700 ml ISP2培养基至2 L三角瓶),置于30 ℃,220 r/min的恒温摇床培养7 d,共得到发酵液50 L。
-
菌株培养7 d后,用等体积的乙酸乙酯萃取3次,合并乙酸乙酯提取液,减压浓缩得粗浸膏9.8 g。粗浸膏先经凝胶柱分离,二氯甲烷:甲醇(1∶1)的混合溶剂进行洗脱,得到组份Fr.1~Fr.7。
组份Fr.4经正相中压柱色谱分离(二氯甲烷:甲醇100:0~0:100),得到组份Fr.4a~Fr.4j。组份Fr.4d再经反相中压柱色谱分离(10%~100%乙腈水),得到组份Fr.4d1~Fr.4d8,Fr.4d2和Fr.4d3用反相半制备HPLC纯化(35%甲醇水,YMC C18),分别得到化合物1 (12 mg, tR=26 min)和2 (47.6 mg, tR=30 min);Fr.4d6用反相半制备HPLC纯化(49%甲醇水,YMC C18),得到化合物3 (8.4 mg, tR=23 min)和4 (2.0 mg, tR=30 min);组份Fr.4g再经凝胶柱纯化,洗脱剂为正己烷:二氯甲烷:甲醇(4∶5∶1),得到组份Fr.4g1~Fr.4g11,其中Fr.4g3用反相半制备HPLC纯化(25%乙腈水,YMC C18),得到化合物5 (1.2 mg, tR=18 min)。
组份Fr.7经反相中压柱色谱分离(10%~100%乙腈水),得到组份Fr.7A~Fr.7D。组份Fr.7A用反相半制备HPLC纯化(20%乙腈水,YMC C18),得到化合物6 (18 mg, tR=19 min)和7 (3.4 mg, tR=25 min);组份Fr.7B经正相中压柱分离(石油醚:丙酮100:0~0:100),得到组份Fr.7B1~Fr.7B9,Fr.7B4用反相半制备HPLC纯化(88%乙腈水,Atlantis Prep T3),得到化合物8 (12 mg, tR=23 min)和9 (8.4 mg, tR=26 min)。
-
化合物1:淡黄色固体,ESI-MS显示准分子离子峰m/z 372 [M+Na]+。1H-NMR (600 MHz, DMSO)显示在低场区有吲哚环的特征信号δH 7.00 (1H, s, H-2),7.49 (1H, d, J=8.0 Hz, H-4),7.02 (1H, t, J=7.6 Hz, H-5),7.05 (1H, t, J=7.6 Hz, H-6),7.32 (1H, d, J=8.0 Hz, H-7);4个活泼氢质子信号δH 10.89 (1H, d, J=2.6 Hz, NH-1),7.83 (1H, d, J=3.0 Hz, NH-10),7.62 (1H, d, J=3.0 Hz, NH-13),9.20 (1H, s, OH-19);4个芳香质子信号δH 6.53 (2H, d, J=8.4 Hz, H-17, H-21),6.59 (2H, d, J=8.4 Hz, H-18, H-20),提示分子中有1个对位二取代的苯环;在高场区有两组亚甲基质子信号δH 2.80 (1H, dd, J=14.5, 4.5 Hz, H-8),2.43 (1H, ov, H-8),δH 1.83 (1H, dd, J=13.4, 6.9 Hz, H-15),2.47 (1H, ov, H-15),两个次甲基质子信号δH 4.01 (1H, m, H-9),3.95 (1H, m, H-12)。13C-NMR (150 MHz, DMSO)结合DEPT谱表明其有20个碳信号,2个酮羰基碳δC 166.7,166.2,14个芳香碳,2个亚甲基碳δC 30.0,40.0和2个次甲基碳δC 55.9,55.2。对其碳信号进行归属:δC 118.7 (C-2)、108.9 (C-3)、127.5 (C-3a)、118.4 (C-4)、120.8 (C-5)、124.3 (C-6)、111.3 (C-7)、136.0 (C-7a)、30.0 (C-8)、55.9 (C-9)、166.7 (C-11)、55.2 (C-12)、166.2 (C-14)、40.1 (C-15)、126.4 (C-16)、130.7 (C-17, C-21)、114.9 (C-18, C-20)、156.0 (C-19)。以上数据与文献[7]对比基本一致,故确定为cyclo-(L-Tyr-L-Trp)。
化合物2:淡黄色固体,ESI-MS显示准分子离子峰m/z 274 [M+H]+。1H-NMR (600 MHz, DMSO)发现其与化合物1一样有吲哚环的特征信号δH 7.09 (1H, s, H-2),7.52 (1H, d, J = 7.9 Hz, H-4),6.99 (1H, t, J = 7.8 Hz, H-5),7.02 (1H, t, J = 7.8 Hz, H-6),7.30 (1H, d, J = 7.8 Hz, H-7);3个活泼氨基质子信号δH 10.87 (1H, s, NH-1),δH 8.30 (1H, m, NH-10),7.85 (1H, d, J = 2.9 Hz, NH-13);两组亚甲基质子信号δH 3.21 (1H, m, H-8),3.13 (1H, m, H-8),δH 3.65 (1H, m, H-15),3.05 (1H, m, H-15),两个次甲基质子信号δH 4.87 (1H, m, H-9),4.00 (1H, m, H-12)。13C-NMR (150 MHz, DMSO)结合DEPT谱表明其有14个碳信号,2个酮羰基碳δC 167.2,165.7,8个芳香碳,2个亚甲基碳δC 63.0,30.3和2个次甲基碳δC 57.3,55.5。对其碳信号进行归属:δC 127.6 (C-2)、111.2 (C-3)、136.0 (C-3a)、118.6 (C-4)、120.8 (C-5)、124.0 (C-6)、118.3 (C-7)、109.0 (C-7a)、30.3 (C-8)、57.3 (C-9)、167.2 (C-11)、55.5 (C-12)、165.7 (C-14)、63.0 (C-15)。以上数据与文献[8]对比基本一致,故确定为cyclo-(L-Trp-L-Ser)。
化合物3:白色固体,ESI-MS显示准分子离子峰m/z 261 [M+H]+。1H-NMR (600 MHz, DMSO)提示有2个活泼氢质子信号δH 7.87 (1H, s, NH-8),9.22 (1H, s, OH-4’);1组对位二取代的苯环芳香质子信号δH 7.04 (2H, d, J = 8.2 Hz, H-2’, H-6’),6.63 (2H, d, J = 8.2 Hz, H-3’, H-5’);2个次甲基质子信号δH 4.24 (1H, t, J = 8.2 Hz, H-6),4.03 (1H, dd, J = 9.9, 2.9 Hz, H-9);4组亚甲基质子信号δH 3.42 (1H, m, H-3),3.24 (1H, m, H-3),1.73 (2H, m, H2-4),2.00 (1H, m, H-5),1.41 (1H, m, H-5),2.93 (2H, m, H2-10)。13C-NMR (150 MHz, DMSO)结合DEPT谱表明其有14个碳信号,2个酮羰基碳δC 168.9,165.1,6个芳香碳,2个次甲基碳δC 58.4,56.0以及4个亚甲基碳δC 44.6,34.7,27.8,21.9。对其碳信号进行归属:δC 165.1 (C-1)、44.6 (C-3)、21.9 (C-4)、27.8 (C-5)、58.4 (C-6)、168.9 (C-7)、56.0 (C-9)、34.7 (C-10)、127.0 (C-1’)、130.8 (C-2’, C-6’)、114.8 (C-3’, C-5’)、155.9 (C-4’)。以上数据与文献[9]对比基本一致,故确定为cyclo-(D-Tyr-D-Pro)。
化合物4:白色固体,ESI-MS显示准分子离子峰m/z 311 [M+H]+。1H-NMR (600 MHz, DMSO)显示3个活泼氢质子信号δH 9.30 (1H, s, OH-11),7.84 (2H, t, J = 2.9 Hz, NH-1, NH-4);9个芳香区质子信号:4个归为1组对位二取代苯环δH 6.85 (2H, d, J = 8.5 Hz, H-9, H-13),6.65 (2H, t, J = 8.5 Hz, H-10, H-12),5个归为1组单取代苯环δH 7.20 (1H, t, J = 7.6 Hz, H-18),7.04 (2H, d, J = 6.9 Hz, H-16, H-20),7.28 (2H, t, J = 7.6 Hz, H-17, H-19);2组亚甲基质子信号δH 2.58 (1H, dd, J = 13.6, 5.0 Hz, H-7),2.20 (1H, d, J = 6.5 Hz, H-7),2.19 (2H, dd, J = 13.6, 6.5 Hz, H2-14),2个次甲基质子信号δH 3.95 (1H, m, H-3),3.90 (1H, m, H-6)。13C-NMR (150 MHz, DMSO)结合DEPT谱显示其有18个碳信号,2个酮羰基碳δC 166.2,166.2,12个芳香碳,2个亚甲基碳δC 40.1,38.5和2个次甲基碳δC 55.7,55.4。对其碳信号进行归属:δC 166.2 (C-2)、55.7 (C-3)、166.3 (C-5)、55.4 (C-6)、40.1 (C-7)、126.5 (C-8)、130.8 (C-9, C-13)、115.0 (C-10, C-12)、156.1 (C-11)、38.5 (C-14)、136.7 (C-15)、129.7 (C-16, C-20)、128.2 (C-17, C-19)、126.4 (C-18)。以上数据与文献[10]对比基本一致,故确定为cyclo-(L-Tyr-L-Phe)。
化合物5:白色固体,ESI-MS显示准分子离子峰m/z 277 [M+H]+。1H-NMR (600 MHz, DMSO)显示3个活泼氢质子信号δH 9.22 (1H, s, OH-4’),8.02 (2H, dd, J = 5.6, 2.5 Hz, NH-1, NH-4);4个芳香质子信号δH 6.90 (2H, d, J = 8.2 Hz, H-2’, H-6’),6.64 (2H, d, J = 8.2 Hz, H-3’, H-5’),提示分子中有1个对位二取代苯环;3个次甲基质子信号δH 4.06 (1H, q, J = 3.3 Hz, H-3),3.44 (1H, m, H-6),1.43 (1H, ov, H-8),2个亚甲基质子信号δH 1.43 (1H, m, H-7),1.23 (1H, m, H-7),2.69 (1H, q, J = 13.6, 4.8 Hz, H-11),3.01 (1H, q, J = 13.7, 3.7 Hz, H-11)以及2个末端甲基质子信号δH 0.63 (6H, ov, H3-9, H3-10)。13C-NMR (150 MHz, DMSO)结合DEPT谱显示其有15个碳信号,2个酮羰基碳δC 166.2,167.4,6个芳香碳,2个亚甲基碳δC 43.7,37.7,3个次甲基碳δC 55.7,52.3,21.4以及2个甲基碳δC 22.9,22.8。对其碳信号进行归属:δC 166.2 (C-2)、55.7 (C-3)、167.4 (C-5)、52.3 (C-6)、43.7 (C-7)、21.4 (C-8)、22.9 (C-9)、22.8 (C-10)、37.7 (C-11)、125.8 (C-1’)、131.2 (C-2’, C-6’)、114.8 (C-3’, C-5’)、156.4 (C-4’)。以上数据与文献[11]对比基本一致,故确定为cyclo-(L-Tyr-L-Leu)。
化合物6:白色固体,ESI-MS显示准分子离子峰m/z 259 [M+Na]+。1H-NMR (600 MHz, DMSO)显示有2个活泼氢质子信号δH 5.05 (1H, d, J = 4.5 Hz, OH-4)和4.39 (1H, q, J = 4.0 Hz, OH-13),3个甲基质子信号δH 0.88 (3H, d, J = 6.8 Hz, H3-12),0.98 (3H, s, H3-14)和δH 1.05 (3H, s, H3-15),5对亚甲基质子信号δH 2.14 (1H, m, H-3),1.23 (1H, m, H-3),1.74 (1H, m, H-9),1.60 (1H, m, H-9),1.44 (3H, m, H2-10, H-11),1.32 (1H, d, J = 10.4 Hz, H-11),3.95 (2H, m, H2-13),3个次甲基质子信号δH 1.68 (1H, m, H-2),4.57 (1H, m, H-4),1.77(1H, m, H-8)。13C-NMR (150 MHz, DMSO)结合DEPT谱共显示有15个碳信号,包括4个季碳δC 52.1,150.1,136.7,39.8;3个次甲基碳δC 35.2,69.8,46.4;5个亚甲基碳δC 42.4,23.8,28.7,36.4,57.1以及3个甲基碳δC 13.7,29.1,24.4。对其碳信号进行归属:δC 52.1 (C-1)、35.2 (C-2)、42.4 (C-3)、68.9 (C-4)、150.1 (C-5)、136.7 (C-6)、39.8 (C-7)、46.4 (C-8)、23.8 (C-9)、28.7 (C-10)、36.4 (C-11)、13.7 (C-12)、57.1 (C-13)、29.1 (C-14)、24.4 (C-15)。以上数据与文献[12]对比基本一致,故确定为albaflavenol B。
化合物7:白色结晶固体,ESI-MS显示准分子离子峰m/z 268 [M+H]+。1H-NMR (600 MHz, DMSO)可看出其有13个氢信号,包括5个活泼氢质子信号δH 3.56 (2H, m, NH2),5.49 (1H, s, OH-5’),5.36 (1H, t, J = 4.9 Hz, OH-2’)和5.23 (1H, s, OH-3’),4个连氧次甲基质子信号δH 4.56 (1H, s, H-2’),4.14 (1H, s, H-3’),3.96 (1H, m, H-4’)和5.90 (1H, d, J = 5.8 Hz, H-1’),1组亚甲基信号δH 3.66 (2H, m, H2-5’)以及2个低场区的烯氢质子信号δH 8.37 (1H, s, H-8)和8.21 (1H, s, H-2)。13C-NMR (150 MHz, DMSO)显示其共有10个碳信号,结合DEPT谱可推测有3个芳香季碳δC 149.9,119.8,154.3,2个连氮的芳香次甲基碳δC 151.7,138.6,4个次甲基碳δC 87.8,73.5,70.5,85.7,1个亚甲基碳δC 61.5。对其碳信号进行归属:δC 151.7 (C-2)、149.9 (C-4)、119.8 (C-5)、154.3 (C-6)、138.6 (C-8)、87.8 (C-1’)、73.5 (C-2’)、70.5 (C-3’)、85.7 (C-4’)、61.5 (C-5’)。以上数据与文献[13]对比基本吻合,故确定为β-adenosine。
化合物8:绿色无定型固体,ESI-MS显示准分子离子峰m/z 297 [M+H]+。1H-NMR (600 MHz, MeOD)显示有12个氢信号,包括1个活泼氢质子信号δH 8.37 (1H, s, H-11),1个甲氧基质子信号δH 3.79 (3H, s),1个甲基质子信号δH 1.25 (3H, d, J = 6.4 Hz, H3-13),3个低场区的芳香氢质子信号δH 8.31 (1H, d, J = 7.8 Hz, H-4),6.92 (1H, t, J = 8.0 Hz, H-5)和7.65 (1H, d, J = 8.0 Hz, H-6),以及2个次甲基质子信号δH 4.74 (1H, d, J = 3.2 Hz, H-8)和4.40 (1H, m, H-12),后与文献[14]对比发现其有4个活泼氢质子信号没有显示出来,而根据相关化学位移可确定其是同一个已知化合物。13C-NMR (150 MHz, MeOD)显示有13个碳信号,结合DEPT谱可推测有6个芳香碳δC 123.4,119.4,126.2,128.2,152.5,115.6,2个羰基碳δC 171.8,172.4,而δC 162.1为醛基碳,2个次甲基碳δC 59.4,68.4,1个甲基碳δC 20.5,以及1个甲氧基碳δC 52.9。对其碳信号进行归属:δC 115.6 (C-1)、152.5 (C-2)、128.2 (C-3)、126.2 (C-4)、119.4 (C-5)、123.4 (C-6)、171.8 (C-7)、59.4 (C-8)、172.4 (C-9)、52.9 (C-10)、162.1 (C-11)、68.4 (C-12)、20.5 (C-13)。以上数据与文献[14]对比基本吻合,故确定为N-formylantimyic acid methyl ester。
化合物9:白色粉末状固体,ESI-MS显示准分子离子峰m/z 499 [M+H]+。1H-NMR (600 MHz, DMSO)显示有19个氢信号:δH 8.20 (1H, s, H-11),6.82 (1H, s, H-10),6.32 (1H, dd, J = 10.5, 1.3 Hz, H-3),5.01 (1H, m, H-7),2.99 (1H, dd, J = 2.5, 15.8 Hz, H-8),2.80 (1H, dd, J = 10.3, 15.6 Hz, H-8),2.58 (1H, m, H-4),1.66 (1H, m, H-5),1.65 (3H, s, 2-Me),1.27 (1H, m, H-6),1.25 (1H, m, H-5),1.06 (3H, d, J = 6.5 Hz, 4-Me)和0.95 (3H, d, J = 5.9 Hz, 6-Me)。而13C-NMR (150 MHz, DMSO)结合DEPT谱显示只有14个碳信号:δC 166.0 (C-1),126.8 (C-2),147.4 (C-3),30.7 (C-4),37.6 (C-5),35.1 (C-6),74.5 (C-7),24.0 (C-8),149.4 (C-9),122.9 (C-10),151.3 (C-11),12.7 (2-Me),21.1 (4-Me)和16.2 (6-Me),说明这个化合物可能是一个具有对称结构的二聚体,通过与文献[15]中化合物conglobatin A对比后发现两者波谱数据完全吻合,故最终确定为conglobatin A。
-
自上世纪发现青霉素以来,微生物中活性次生代谢产物一直是药物先导化合物的重要来源之一,据统计1940年—2019年间,科学家从微生物中开发出293种治疗不同疾病的临床药物[16]。但随着研究的深入,很多微生物及其次生代谢产物存在被重复开发和提取分离的问题,加之多重耐药性的产生,迫使人们需要开拓新的制造药物的微生物来源[5-6],而其中极地微生物资源是珍贵而特殊的。来自极地海洋等特殊生态环境的生物往往具有比陆地生物更为丰富的代谢途径和功能基因簇,增加了产生结构新颖且功能独特的次生代谢物的可能性。极地生物以微生物和一些能适应极端条件的海洋生物为主,然而与已报道的大量极地微生物相比,鲜有微生物活性天然产物相关研究报道,因此,极地微生物极具研究价值[17-18]。
笔者以一株采自北极海域海绵共附生放线菌Streptomyces sp. LHW11-07为研究对象,从其发酵浸膏中分离得到9个单体化合物1~9,包括环二肽化合物1~5,倍半萜化合物6,核苷类化合物7,以及两个其他结构类型化合物8和9,其中化合物1和2是首次分离于Streptomyces放线菌,而这些化合物的生物活性还有待进一步探究;本研究进一步丰富了该属放线菌的化学多样性,同时,为高值化开发利用极地微生物这一国家战略资源提供了物质基础和理论依据。
据文献报道,化合物1对所测试的病原性细菌和真菌均具有一定的对抗作用[19],化合物2测试了4种肿瘤细胞均无明显的细胞毒性[20],化合物3对海胆Strongylocentrotus intermedius胚胎具有细胞毒活性[9],化合物5具有抗炎活性并对H1N1和RSV病毒有一定的杀伤作用[21],化合物7作为一种内源性嘌呤核苷,具有降低血压、抑制血小板聚焦、舒张血管、减慢心律等生理活性[22],而化合物9可抑制癌细胞株的增殖,在体外对Trypanosoma brucei brucei GUTat 3.1表现出抗锥虫体活性等[23]。
Study on the secondary metabolites from a polar marine sponge associated actinomyces Streptomyces sp. LHW11-07
doi: 10.12206/j.issn.2097-2024.202206081
- Received Date: 2022-06-18
- Rev Recd Date: 2022-07-16
- Available Online: 2022-11-28
- Publish Date: 2022-11-25
-
Key words:
- polar region /
- sponge /
- actinomyces /
- secondary metabolite
Abstract:
| Citation: | LU Jingrong, LIU Yawei, DAI Ying, LIN Houwen, YANG Fan. Study on the secondary metabolites from a polar marine sponge associated actinomyces Streptomyces sp. LHW11-07[J]. Journal of Pharmaceutical Practice and Service, 2022, 40(6): 499-504. doi: 10.12206/j.issn.2097-2024.202206081 |







 DownLoad:
DownLoad: